Researchers develop simple technique to visualize atomic-scale structures
Researchers at the California Institute of Technology (Caltech) have devised a new technique -- using a sheet of carbon just one atom thick -- to visualize the structure of molecules. The technique, which was used to obtain the first direct images of how water coats surfaces at room temperature, can also be used to image a potentially unlimited number of other molecules, including antibodies and other biomolecules.
A paper describing the method and the studies of water layers appears in the September 3 issue of the journal Science.
"Almost all surfaces have a coating of water on them," says James Heath, the Elizabeth W. Gilloon Professor and professor of chemistry at Caltech, "and that water dominates interfacial properties"—properties that affect the wear and tear on that surface. While surface coatings of water are ubiquitous, they are also very tough to study, because the water molecules are "in constant flux, and don't sit still long enough to allow measurements," he says.
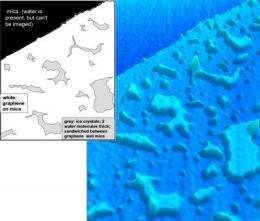
Quite by accident, Heath and his colleagues developed a technique to pin down the moving molecules, under room-temperature conditions. "It was a happy accident—one that we were smart enough to recognize the significance of," he says. "We were studying graphene on an atomically flat surface of mica and found some nanoscale island-shaped structures trapped between the graphene and the mica that we didn't expect to see."
Graphene, which is composed of a one-atom-thick layer of carbon atoms in a honeycomb-like lattice (like chicken wire, but on an atomic scale), should be completely flat when layered onto an atomically flat surface. Heath and his colleagues—former Caltech graduate student Ke Xu, now at Harvard University, and graduate student Peigen Cao—thought the anomalies might be water, captured and trapped under the graphene; water molecules, after all, are everywhere.
To test the idea, the researchers conducted other experiments in which they deposited the graphene sheets at varying humidity levels. The odd structures became more prevalent at higher humidity, and disappeared under completely dry conditions, leading the researchers to conclude that they indeed were water molecules blanketed by the graphene. Heath and his colleagues realized that the graphene sheet was "atomically conformal"—it hugged the water molecules so tightly, almost like shrink wrap, that it revealed their detailed atomic structure when examined with atomic force microscopy. (Atomic force microscopes use a mechanical probe to essentially "feel" the surfaces of objects.)
"The technique is dead simple—it's kind of remarkable that it works," Heath says. The method, he explains, "is sort of like how people sputter carbon or gold onto biological cells so they can image them. The carbon or gold fixes the cells. Here, the graphene perfectly templates the weakly adsorbed water molecules on the surface and holds them in place, for up to a couple of months at least."

Using the technique, the researchers revealed new details about how water coats surfaces. They found that the first layer of water on mica is actually two water molecules thick, and has the structure of ice. Once that layer is fully formed, a second, two-molecule-thick layer of ice forms. On top of that, "you get droplets," Heath says. "It's truly amazing that the first two adsorbed layers of water form ice-like microscopic islands at room temperature," says Xu. "These fascinating structures are likely important in determining the surface properties of solids, including, for example, lubrication, adhesion, and corrosion."
The researchers have since successfully tested other molecules on other types of atomically flat surfaces—such flatness is necessary so the molecules don't nestle into imperfections in the surface, distorting their structure as measured through the graphene layer. "We have yet to find a system for which this doesn't work," says Heath. He and his colleagues are now working to improve the resolution of the technique so that it could be used to image the atomic structure of biomolecules like antibodies and other proteins. "We have previously observed individual atoms in graphene using the scanning tunneling microscope," says Cao. "Similar resolution should also be attainable for graphene-covered molecules."
"We could drape graphene over biological molecules—including molecules in at least partially aqueous environments, because you can have water present—and potentially get their 3-D structure," Heath says. It may even be possible to determine the structure of complicated molecules, like protein-protein complexes, "that are very difficult to crystallize," he says.
Whereas the data from one molecule might reveal the gross structure, data from 10 will reveal finer features—and computationally assembling the data from 1,000 identical molecules might reveal every atomic nook and cranny.
If you imagine that graphene draped over a molecule is sort of like a sheet thrown over a sleeping cat on your bed, Heath explains, having one image of the sheet-covered lump—in one orientation—"will tell you that it's a small animal, not a shoe. With 10 images, you can tell it's a cat and not a rabbit. With many more images, you'll know if it's a fluffy cat—although you won't ever see the tabby stripes."
More information: "Graphene Visualizes the First Water Adlayers on Mica at Ambient Conditions," Science, Sept 3 2010.
Provided by California Institute of Technology