Using 'dark channel' fluorescence, scientists explain how biochemical substances carry out their function
Spectroscopic techniques are among the most important methods by which scientists can look inside materials. They exploit the interaction of light waves with a given sample.
Now, using X-ray absorption spectroscopy, researchers from Helmholtz-Zentrum Berlin fur Materialien und Energie (HZB) have observed the moving of electric charges from solute to solvent - so-called electron transfer. They can even make assertions on the temporal sequence of this process. As one example, they can find out how solute biochemical substances carry out their microscopic functions in their natural environment at room temperature and normal pressure. Until recently, studying such systems by soft X-ray radiation has not been possible. The HZB group led by Emad Aziz reports on this in Nature Chemistry, with their article highlighted in the online pre-issue from 8 August.
The group studied the X-ray absorption spectra of iron ions in both iron chloride and organic compounds such as haemin, the active centre of blood component haemoglobin, and analyzed the hitherto inexplicable negative peak (dip) in the spectra.
In X-ray absorption spectroscopy, monochromatic X-ray light interacts with the sample. When the energy of the incident light exactly matches the energy transfer in the molecule, electrons can be excited out of their ground state into a higher energy state. As they return to their original state, the added energy is released again, as an emission of fluorescent light for example. By recording this fluorescent light, scientists gain an insight into the electron orbital configuration of atoms and molecules.
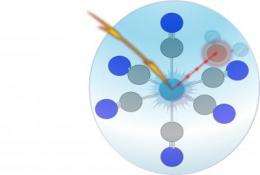
By making measurements using synchrotron light at the X-ray source BESSY II, Emad Aziz and his colleagues discovered that certain solute substances emit no fluorescent light after excitation. The negative peak that appeared in the spectrum was evidence that the return to ground state took place without radia-tion, through a so-called "dark channel".
This happens because interactions between molecules in the sample and in the solvent produce common orbitals. The excited electrons are pushed into these orbitals. "This works because the molecular orbitals of the iron and water ions come very close spatially and their energies match very well," explains Emad Aziz, head of a junior research group at HZB. The electrons remain in this new state longer than they would in a normal molecular orbital. Their energy state therefore prevents the emission of the normally expected fluorescent light.
Dips in the spectrum thus give a clue as to the kind of interplay between the sample and the solvent. One could use this process to examine how much the solvent contributes towards the function of biochemical systems such as pro-teins, for example.
Ultrafast processes such as charge transfer have only been observable with enormous effort using conventional methods. Now, HZB researchers have found a way to explain the dynamics of this process using a simple model. "We can observe where the charges migrate to, and we can see that this happens within a few femtoseconds," Emad Aziz stresses. The result also has major repercus-sions for the interpretation of X-ray absorption spectra in general.
For their experiments, the group used a specially developed flow cell that also allows them to study biological samples by X-ray in their natural environment - that is in dissolved form.
More information: Paper: DOI:10.1038/NCHEM.768
Provided by Helmholtz Association of German Research Centres