'Security guard' zinc is off-duty in diabetes
(PhysOrg.com) -- In type 2 diabetes, a protein called amylin forms dense clumps that shut down insulin-producing cells, wreaking havoc on the control of blood sugar. But in people without diabetes, amylin doesn't misbehave; it actually pitches in to help with blood sugar regulation. What accounts for the difference?
It's all about the company amylin keeps, new research by Ayyalusamy Ramamoorthy and colleagues at the University of Michigan suggests. In the presence of zinc, amylin is mild-mannered; without zinc, it runs amok. The research will appear in the July 7 issue of the Journal of the American Chemical Society.
"We found that one of the likely factors stopping amylin from attacking the insulin-producing islet cells of the pancreas is zinc, which normally is found in high amounts in these cells, but is deficient in people with type 2 diabetes," said Ramamoorthy, U-M professor of chemistry and of biophysics. "By understanding what stops amylin from attacking islet cells in normal people, we hope we'll be able to understand how it is attacking them in people with diabetes."
The new research suggests that in healthy cells, zinc acts like a security guard at a rock concert, whose job is keeping fans from turning troublesome and destructive. In molecular terms, zinc prevents amylin—also known as Islet Amyloid Polypeptide (IAPP)—from forming harmful clumps similar to those found in Alzheimer's, Parkinson's, Huntington's and various other degenerative diseases.
But in the zinc-starved cellular environment of someone with type 2 diabetes, amylin has no watchful guard to rein it in. It's free to band together other amylin molecules in the molecular equivalent of a gang.
Ramamoorthy and colleagues investigated zinc's role in amylin aggregation using several methods, including NMR (nuclear magnetic resonance) spectroscopy.
"We wanted to see how zinc was affecting the structure of amylin," said Ramamoorthy, "and NMR is the technique we use to do this."
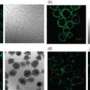
The researchers obtained images of amylin with and without zinc present and saw that zinc binds to a particular side chain, disrupting the structure of amylin in the process. Other experiments showed that—at zinc concentrations typical of those found in the environment in which aggregation occurs—this binding significantly slows aggregation.
Other experiments underway in Ramamoorthy's laboratory are aimed at rounding out the picture by looking at interactions between zinc and insulin and between insulin and amylin.
"Ultimately, we want to understand how the whole scenario leads to type 2 diabetes," Ramamoorthy said.
More information: pubs.acs.org/journal/jacsat
Provided by University of Michigan