Crystal defect shown to be key to making hollow nanotubes
(PhysOrg.com) -- Scientists have no problem making a menagerie of nanometer-sized objects -- wires, tubes, belts, and even tree-like structures. What they sometimes have been unable to do is explain precisely how those objects form in the vapor and liquid cauldrons in which they are made.
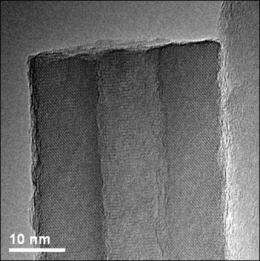
Now a team led by University of Wisconsin-Madison chemist Song Jin, writing this week (April 23, 2010) in the journal Science, shows that a simple crystal defect known as a "screw dislocation" drives the growth of hollow zinc oxide nanotubes just a few millionths of a centimeter thick.
The finding is important because it provides new insight into the processes that guide the formation of the smallest manufactured structures, a significant challenge in nanoscience and nanotechnology. "We think that this work provides a general theoretical framework for controlling nanowire or nanotube growth without using metal catalysts that can be generally applicable to many materials," says Jin, a UW-Madison professor of chemistry.
Such materials and the Lilliputian structures scientists sculpt have already found broad applications in such things as electronics, solar power, battery and laser technology, and chemical and biological sensing. By further expanding the theory of how the tiny structures form, it should now be possible for scientists to develop new methods to mass produce nano-sized objects using a variety of different materials.
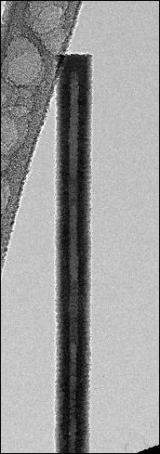
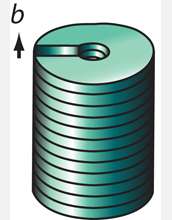
The method described by Jin and his colleagues depends on what scientists call a screw dislocation. Dislocations are fundamental to the growth and characteristics of all crystalline materials. As their name implies, these defects prompt the creation of spiral steps on an otherwise flawless crystal face. As atoms alight on the crystal surface, they form a structure strikingly similar in appearance to the spiral ramps of multistory parking structures. In earlier work, Jin and his research group showed that screw dislocations drive the growth of one-dimensional nanowire structures that looked like tiny pine trees. That, says Jin, was a critical clue to understanding the kinetics of spontaneous nanotube growth.
The key to understanding how to harness the defect to make nanostructures in a rational way, Jin explains, is knowing that as atoms collect on a surface of a dislocation spiral, strain associated with screw dislocations builds up in the tiny structures they create.
It turns out that "making the structure hollow and making it twist are two good ways of relieving such strain and stress," Jin explains. "In some cases, the large screw dislocation strain energy contained within the nanomaterial dictates that the material hollow out its center around the dislocation, thus resulting in the spontaneous formation of nanotubes."
The phenomenon described in the new Wisconsin work differs in significant ways from traditional mechanisms of making hollow nanostructures. Scientists now use templates to "mold" nanotubes or, alternatively, a diffusion process to convert one material into another with a hollow core. Carbon nanotubes are made, essentially, by rolling up a single honeycomb-patterned layer of carbon atoms.
The phenomena described by the Wisconsin team, Jin adds, should apply to materials beyond zinc oxide: "The understanding of the formation of nanotubes will certainly help us to understand related phenomena in other materials."
Refined, the new knowledge could ultimately be turned to the large scale, low cost production of nanomaterials for a wide range of applications. Most promising, says Jin, is the area of renewable energy where large amounts of such materials can be deployed to convert sunlight to electricity, and provide new raw materials for battery electrodes and thermoelectric devices.
Provided by University of Wisconsin-Madison