Photosynthesis: a new source of electrical energy
French scientists have transformed the chemical energy generated by photosynthesis into electrical energy. They thus propose a new strategy to convert solar energy into electrical energy in an environmentally-friendly and renewable manner. The biofuel cell thus developed could also have medical applications. These findings have just been published in the journal Analytical Chemistry.
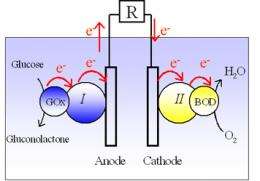
Photosynthesis is the process by which plants convert solar energy into chemical energy. In the presence of visible light, carbon dioxide (CO2) and water (H20) are transformed into glucose and O2 during a complex series of chemical reactions. Researchers at the Centre de Recherche Paul Pascal (CNRS) developed a biofuel cell that functions using the products of photosynthesis (glucose and O2) and is made up of two enzyme-modified electrodes.
The cell was then inserted in a living plant, in this case a cactus. Once the electrodes, highly sensitive to O2 and glucose, had been implanted in the cactus leaf, the scientists succeeded in monitoring the real-time course of photosynthesis in vivo. They were able to observe an increase in electrical current when a desk lamp was switched on, and a reduction when it was switched off. During these experiments, the scientists were also able to make the first ever observation of the real-time course of glucose levels during photosynthesis. This method could offer a new means of better understanding the mechanisms of photosynthesis.
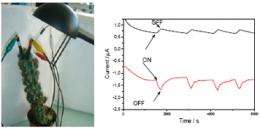
Furthermore, the researchers showed that a biofuel cell inserted in a cactus leaf could generate power of 9 W per cm2. Because this yield was proportional to light intensity, stronger illumination accelerated the production of glucose and O2 (photosynthesis), so more fuel was available to operate the cell. In the future, this system could ultimately form the basis for a new strategy for the environmentally-friendly and renewable transformation of solar energy into electrical energy.
Alongside these results, the initial objective of this work was to develop a biofuel cell for medical applications. This could then function autonomously under the skin (in vivo), drawing chemical energy from the oxygen-glucose couple that is naturally present in physiological fluids. It could thus provide power for implanted medical devices such as, for example, autonomous subcutaneous sensors to measure glucose levels in diabetic patients.
More information: From Dynamic Measurements of Photosynthesis in a Living Plant to Sunlight Transformation into Electricity, Victoria Flexer, Nicolas Mano, Analytical Chemistry, 15 February 2010.
Provided by CNRS
















