Researchers find key interaction that controls telomeres
In the dominoes that make up human cells, researchers at the University of Michigan Comprehensive Cancer Center have traced another step of the process that stops cells from becoming cancerous.
It starts with the enzyme telomerase, which affects the caps, or telomeres, at the end of a chromosome. Telomeres shorten over time. But telomerase prevents this from happening, making the cell immortal. If cancer is triggered in the cell, the presence of telomerase leads to the growth of the cancer.
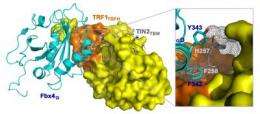
Telomerase is kept in control by the protein TRF1, which keeps the telomeres operating correctly. But another protein, Fbx4, can bind to TRF1 and degrade it, causing the telomeres to lengthen.
Now, researchers have discovered, a third protein, TIN2, can step in and override Fbx4 by binding to TRF1 first and preventing Fbx4 from attaching to it.
This finding paves the way for developing a drug that acts like TIN2, keeping everything in check and stopping the first domino from falling.
Results of the study appear in the Feb. 16 issue of Developmental Cell.
"In 90 percent of cancers, no matter what caused the cancer to form, it needs telomerase activity to maintain the cell. Without telomerase, the cell will die. Our work is key to understanding a detailed mechanism for how these molecules interact and how to design a drug to block Fbx4," says senior author Ming Lei, Ph.D., assistant professor of biological chemistry at the University of Michigan Medical School.
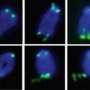
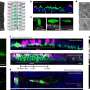
The researchers found that the location in the molecule where Fbx4 binds to TRF1 overlaps with where TIN2 binds to TRF1. Where both Fbx4 and TIN2 are present, the TIN2 wins out and binds to the TRF1 first. This blocks Fbx4 from binding to the TRF1, thereby stabilizing TRF1 and keeping the telomere length in control.
The researchers are now looking at peptides that mimic TIN2's binding to TRF1, in order to block Fbx4. The work is still in preliminary stages and no new therapies are being tested in patients.
If a drug is discovered, it could impact all cancer types. Currently, molecularly targeted therapies address a pathway or gene that's involved in only specific types of cancer. But telomerase is involved in all types of cancer.
"If we find a drug that can inhibit telomerase activity in any fashion, that could be a universal cancer drug," says Lei, a Howard Hughes Medical Institute Early Career Scientist.
More information: Developmental Cell, Vol. 18, No. 2, pp. 214-225
Provided by University of Michigan