Carbenes: New molecules have wide applications
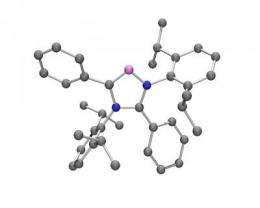
Researchers at the University of California, Riverside have created in the laboratory a class of carbenes, highly reactive molecules, used to make catalysts - substances that facilitate chemical reactions. Until now, chemists believed these carbenes, called "abnormal N-heterocyclic carbenes" or aNHCs, were impossible to make.
Carbenes are made up of unusual carbon atoms and are usually unstable in nature. They attach themselves to metals to form metal-carbene complexes that serve as efficient catalysts used widely in the pharmaceutical industry.
The metal-carbene complexes are formed in two ways: (a) the complex is created in one step, without first preparing carbene independently, and (b) a metal and an independent carbene are brought together to make the complex.
Most often the metal used in a metal-carbene complex is rhodium, gold, platinum or palladium - all of which are very expensive and, in some cases, even toxic. To bring down the cost of catalysts, when possible, carbenes are used independently (without metals) in many chemical reactions.
Until now, aNHCs have been used as only metal-carbene complexes, never independently. Chemists had assumed that aNHCs cannot exist freely, which made them impossible to make.
Now UC Riverside's Guy Bertrand, a distinguished professor of chemistry, and colleagues have challenged that assumption by successfully creating aNHCs that are metal-free and can be used to make any desired complex.
"Many chemical species are believed to be unstable because they do not obey the rules we learned at school, and consequently nobody tries to make them," said Bertrand, who led the research project. "The role of scientists, however, is to challenge former hypotheses. That is just what we did in the case of the aNHCs, and we were successful.
"The aNHCs are stable at room temperature both in the solid state and in solution, which means their application as metal-free catalysts is extremely wide, greatly benefiting industry by making possible scores of new chemical reactions."
Results of the study appear in the Oct. 23 issue of Science.
"This study, reporting the synthesis and characterization of an entirely different class of metal-free NHCs, could open new horizons and have a huge impact on the field of catalysis," said John Schwab, who oversees organic synthesis grants at the National Institutes of Health's National Institute of General Medical Sciences. "The potential applications to drug discovery and manufacture are exciting, since catalytic processes can help keep costs in check and be environmentally friendly, to boot."
Bertrand is interested in making aNHCs commercially available. "We hope many chemists in the world will use these carbenes and find some new applications," he said.
The UCR Office of Technology Commercialization has filed a patent application on the technology and is currently seeking partners in industry interested in developing the technology commercially.
Source: University of California - Riverside (news : web)

















