Scientists visualize assembly line gears in ribosomes, cell's protein factory
Even as research on the ribosome, one of the cell's most basic machines, is recognized with a Nobel Prize, scientists continue to achieve new insights on the way ribosomes work.
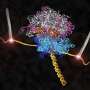
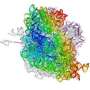
Ribosomes are factories inside cells where messages coming from genes are decoded and new proteins pieced together on an assembly line. For the first time, scientists have a detailed picture of the ribosome trapped together with elongation factor G (EF-G), one of the enzymes that nudges the assembly line to move forward.
The results are published in the Oct. 16 issue of Science magazine.
A team led by Venki Ramakrishnan at the MRC Laboratory of Molecular Biology in Cambridge, England analyzed crystals of the ribosome bound to EF-G using X-rays, and used the X-ray data to determine the molecular structure.
One member of the team, Christine Dunham, PhD, recently joined Emory University School of Medicine's Department of Biochemistry as an assistant professor.
The scientists obtained crystals by growing heat-tolerant bacteria found at thermal vents at the bottom of the ocean. They purified the ribosomes from the bacteria and then added polymers carefully selected to coax the ribosomes into lining up and forming crystals. In addition, they included an antibiotic - fusidic acid - which traps EF-G on the ribosome.
Previous efforts to crystallize ribosomes together with EF-G led to EF-G being displaced from the crystals. Dunham says the team was able to visualize the ribosome bound to EF-G only by shaving off part of the ribosome. Modifying the bacterial gene that encoded a part of the ribosome with a "very strange and elongated protein shape" allowed crystals that included EF-G to form.
Dunham says details from the new structure show that EF-G interacts closely with parts of the ribosome, suggesting how it moves the assembly line forward without slipping out of frame. In addition, it paves the way for studying interactions between the ribosome and other proteins similar to EF-G that fit into the same spot.
In her own research, Dunham is examining how viruses such as HIV, upon hijacking ribosomes, use special tricks that cause the assembly line to slip, as well as how other antibiotics and toxin proteins interact with parts of the ribosome.
More information: Y.G. Gao, M. Selmer, C.M. Dunham, A. Weizlbaumer, A.C. Kelley and V. Ramakrishnan. The structure of the ribosome with elongation factor G trapped in the post-translocational state. Science XX, YY (2009)
Source: Emory University (news : web)