Alzheimer's research yields potential drug target
Scientists at UC Santa Barbara and several other institutions have found laboratory evidence that a cluster of peptides may be the toxic agent in Alzheimer's disease. Scientists say the discovery may lead to new drugs for the disease.
In an article published this week in Nature Chemistry, the researchers explain the process in which the toxic Amyloid Beta 42 peptides aggregate, and outline the new technology they use to study these peptides. The findings come out of the laboratory of Michael T. Bowers, professor of chemistry and biochemistry at UCSB.
"We believe that we have put a face, a structure, on the molecular assembly that is responsible for Alzheimer's disease," said Bowers. His research group used an innovative technology called ion mobility-based mass spectroscopy, a method that allows researchers to investigate the structure, aggregation, and energetics of protein and peptide systems.
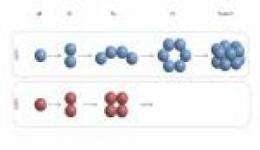
The Amyloid Beta (AB) 42 peptide is clipped from a much larger protein, the amyloid precursor protein (APP), and is composed of 42 amino acid residues. A second peptide, AB40, is 10 times more abundant than AB42 in healthy human brains and is also clipped from APP. It is identical to AB42 except it is missing the last two amino acids. Both peptides aggregate, but AB42 more so than AB40.

AB40 never grows beyond a tetramer -- a cluster of four AB40 peptides. As a consequence, it is nontoxic. By contrast, AB42 grows to form rings of six units each. Two of these "six-mer" rings stack to form a dodecamer, or "twelve-mer," according to Bowers, and then the aggregation stops. These dodecamer clusters are long-lived, but may eventually rearrange to form so-called B-sheet structures, which lead to the large fibrils that form the plaques found in the brains of those with Alzheimer's disease and other neurodegenerative diseases.
In related studies, transgenic mice, implanted with the gene that expresses human APP (and hence able to form AB42 in their brains), are found to quickly develop memory deficits -- as if they have Alzheimer's disease. Since mice have a much faster metabolism than humans, the disease progresses more quickly. Of importance is the fact that the only AB species found in the brains of the transgenic mice correlates with the dodecamer of AB42 characterized in the Bowers lab experiments. These two pieces of data together strongly implicate the dodecamer of AB42 as the toxic agent in Alzheimer's disease.
"Our group, along with our collaborators, are searching for drug candidates that can prevent AB42 from aggregating to form the toxic dodecamer," said Bowers. "While it is early in the search, we are hopeful good candidates can be found. As a consequence, there is a need to find an early marker for Alzheimer's disease so that we can use these drugs to radically slow down the disease progression."
Bowers explained that this research method is new, but is gaining acceptance in the biological community. He said that to fully understand the disease, effects of the oligomerization process would have to be observed at the cellular level, however.
"These latest results are a very hopeful thing," said Bowers. "I'm more hopeful now than I have ever been that we can make some real progress on this terrible disease."
The National Institutes of Health funded the study. When Bowers first received the funding, he explained: "In biology, structure and function are tightly coupled. When it became clear that small soluble oligomers were most probably the toxic agents in Alzheimer's disease, I realized our ion mobility methods could contribute, since we could measure the oligomer distribution and shapes of these peptides for the first time."
Source: University of California - Santa Barbara (news : web)















