A penny for your prions: Researchers study link between copper, mad cow disease
(PhysOrg.com) -- North Carolina State University researchers have discovered a link between copper and the normal functioning of prion proteins, which are associated with transmissible spongiform encephalopathy diseases such as Cruetzfeldt-Jakob in humans or "mad cow" disease in cattle.
Their work could have implications for patients suffering from these diseases, as well as from other prion-related diseases such as Alzheimers or Parkinson's.
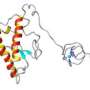
Prion proteins, or PrPs, are commonly found in brain tissue and throughout the central nervous system. In humans or animals with prion diseases, these proteins deform and aggregate, creating clumps of PrPs that interfere with the nervous system's ability to function normally. A team of NC State physicists, led by Miroslav Hodak and Jerry Bernholc, has found that when PrPs bind with copper in the human body, their structure becomes more stable and less likely to misfold or aggregate.
"We believe that a prion protein's normal function is to serve as a copper buffer in the human body, binding with copper ions and keeping those ions from damaging human tissue," Hodak says. "We wanted to determine whether this was the normal function of the prion, and then look at how that binding affected the prion's structure."
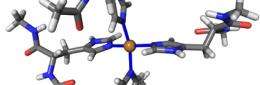
The researchers created a 3-D model of the PrP using supercomputers at Oak Ridge National Laboratories. With the model, they determined that PrPs can bind up to four copper ions apiece, depending on the concentration of copper present. They also found that when the PrPs bind to the copper ions, the structure of the protein changes, becoming more stable.
Their results are published online this week in Proceedings of the National Academy of Sciences.
"Prion proteins are unusual in that half of the protein has a well-defined structure, but the other half of it - where the binding occurs - is a flexible, random tangle," Hodak says. "When we looked at the so-called 'random' portion of the PrP where that binding occurs, we found that the copper ions lend stability to the overall protein. This stability may play a role in preventing PrPs from misfolding or aggregating - which indicates that with prion diseases, copper binding may be beneficial."
More information: "Cu2+ Binding to the Prion Protein: Functional Implications and the Role of Copper"
Authors: Miroslav Hodak, and Robin Chisnell, North Carolina State University; Wenchang Lu and Jerry Bernholc, North Carolina State University and Oak Ridge National Laboratories; Published: Online the week of June 22, 2009, in Proceedings of the National Academy of Sciences
Source: North Carolina State University (news : web)