'Taco shell' protein: Orientation of middle man in photosynthetic bacteria described
(PhysOrg.com) -- Researchers at Washington University in St. Louis have figured out the orientation of a protein in the antenna complex to its neighboring membrane in a photosynthetic bacterium, a key find in the process of energy transfer in photosynthesis.
Robert Blankenship, Ph.D., Markey Distinguished Professor of Biology and Chemistry in Arts & Sciences, led a team that for the first time combined chemical labeling with mass spectroscopy to verify the orientation. The team also included Michael Gross, Ph.D., WUSTL Professor of Chemistry, Immunology and Medicine, and Chemistry graduate students Jianzhong Wen and Hao Zhang. A paper describing this work appeared recently in the Proceedings of the National Academy of Sciences USA.
In green sulfur bacteria, which live in extremely dim environments with scarce visible light, the membrane-attached Fenna-Matthews-Olson (FMO) antenna protein serves as a sort of wire connecting the large peripheral chlorosome antenna complex with the organism's reaction center.
These bacteria are related to extreme heat-loving bacteria that live at thermal vents on the ocean floor. Their antenna systems are much larger and more pronounced than those of other bacteria to take advantage of whatever geothermal light they can harvest.
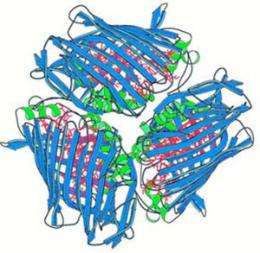
Blankenship fondly refers to the FMO protein as the "taco shell protein" because of its structure: its ribbon-like backbone wraps around three clusters of seven chlorophylls, just like a taco shell around ground beef. The structure also is referred to as trimeric because of the three clusters.
The taco shell is a sort of "middleman" in the antenna system, sandwiched in between a larger antenna and a complex called the reaction center, where all the electron transfer chemistry takes place.
Most of the absorption of light is carried out by a complex called the chlorosome that then transfers the energy to the trimeric protein that in turn transfers to the reaction center.
Photosynthesis transforms light, carbon dioxide and water into chemical energy in plants and some bacteria. The wavelike characteristic of this energy transfer process can explain its extreme efficiency, in that vast areas of phase space can be sampled effectively to find the most efficient path for energy transfer.
"We used a combination of tried and true methods, but two that hadn't been used together in the past," said Blankenship. "The surface of the protein has various amino acid residues, and some of those are reactive to the chemical probe we added into the system. The surface residues that react to the probe are then labeled, and we isolate the protein and characterize where the label is in the protein by using mass spectroscopy. That's a kind of footprinting analysis."
This allowed the researchers to determine how the protein is oriented on the membrane. The footprinting revealed that the energy will flow from the outer part of the antenna, through the mid-part and into the membrane where the reaction center is located.
"The bacteria use the energy of the pigments as a kind of ladder," he said. "As it goes on this ladder, it goes to lower and lower energy states and is guided down to the lowest energy state. That's the funneling effect — the physical guiding of the energy to the reaction center. By knowing exactly how this orientation is on the membrane, we determined the funneling property in a more precise way."
The trimeric protein — the taco shell protein — has a symmetry axis down the middle. The protein lays on the membrane with the symmetry axis perpendicular to the membrane. The combination of labeling and mass spectroscopy enabled the researchers to determine which side of the protein was up and which side was down.
"It turns out that the side that is down is the one that has the pigment with the lowest energy, which is exactly what you want to facilitate the energy transfer," Blankenship said. "That's what you would imagine if you designed it yourself."
The biochemical aspects of the project were done in the Blankenship lab, while the mass spectrometry analysis was done in the WUSTL National Institutes of Health Mass Spectrometry Resource Facility that is directed by Gross.
Provided by Washington University in St. Louis (news : web)


















