A water splitter with a double role
(PhysOrg.com) -- There is a lot of hope invested in hydrogen, but it also presents some problems. It is energy-rich, clean and, as a constituent of water, of almost unlimited availability. However, so far it has been difficult to access it. Scientists at the Max Planck In-stitute of Colloids and Interfaces have now found a simple, low-cost way to produce hydrogen.
They extract this energy source from water by irradiating it with sunlight and using a carbon nitride as an inexpensive photo catalyst. Up to now this reaction has required organometal compounds and inorganic semiconductors combined with expensive precious metals, such as platinum. (Nature Materials, January 2009)
Hydrogen is seen as the energy source of the future. There is around three times as much energy in a kilogramme of hydrogen as in a kilogramme of crude oil. In ad-dition, extracting energy from hydrogen in fuel cells, for example, creates no pol-lutants, only water. However, hydrogen is only present on the Earth in compounds such as water. Hydrogen must be in its pure form to create energy, and it must be produced with regenerative energy sources such as sunlight.
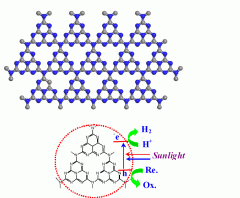
Scientists at the Max Planck Institute of Colloids and Interfaces have succeeded in taking a step in this direction with, surprisingly, one of the oldest polymers known to chemists. They used a carbon nitride, manufactured for the first time by Justus Liebig in 1834 and which he called "melon", to create hydrogen from water with the aid of sunlight. "The special thing about carbon nitride is that it is stable in water, even under extremely acidic or alkali conditions. Apart from that, it is very easy and inexpensive to produce," says Xinchen Wang, whose research group carried out the experiments in collaboration with the University of Tokyo and Fuzhou University in China.
Carbon nitride uses sunlight to extract hydrogen from water. A substance that chemists call a sacrificial reagent absorbs the oxygen from the water. The clever trick here is that the chemists in Potsdam did not need to use a precious metal like platinum. The traditional processes require precious metals, in addition to a semiconductor acting as an aerial for the sunlight, to catalyse the production of hydrogen. The carbon nitride does both jobs at the same time and furthermore acts as a particularly stable organic semiconductor that is much easier to produce than the inorganic materials normally used.
However, only four micromoles of hydrogen per hour bubbled up out of the re-searchers' reaction vessel. "Our yield is therefore not as high as that achieved with the established methods," says Xinchen Wang. "But we have shown that it is, in principle, possible to manufacture hydrogen with just a single organic substance as an additive." When the researchers added the normal quantities of platinum as a catalyst, the yield increased substantially - by a factor of seven. This means that there is not much more benefit compared to the existing methods, as they work with similar quantities of precious metals as catalysts. Wang and his colleagues are therefore trying to make the carbon nitride more efficient by increasing its active surface area.
"It would be ideal for technical applications if we could split water into hydrogen and elementary oxygen in one step," says Wang. Then chemists would not need a sacrificial reagent to absorb the oxygen. This means, however, that they would have to oxidise the oxygen, in the same way as plants can when they photosynthesise. The researchers' calculations have shown that this should also be possible with carbon nitride as the only additive. In experiments, though, they have needed an additional catalyst.
Wang's scientists are now working on a configuration to combine the production of hydrogen and oxygen. If they are successful, the process of water splitting will be perfect and hydrogen will be a step closer to fulfilling its role as an important source of energy.
More information: Xinchen Wang, Kazuhiko Maeda, Arne Thomas, Kazuhiro Takanabe, Gang Xin, Johan M. Carlsson, Kazunari Domen, Markus Antonietti, A metal-free, polymeric photo catalyst for hydrogen production from water under visible light, Nature Materials, 2009, 8, 76-80
Provided by Max Planck Institute of Colloids and Interfaces