'2-faced' Bioacids Put a New Face on Carbon Nanotube Self-Assembly

Nanotubes, the tiny honeycomb cylinders of carbon atoms only a few nanometers wide, are perhaps the signature material of modern engineering research, but actually trying to organize the atomic scale rods is notoriously like herding cats. A new study* from the National Institute of Standards and Technology and Rice University, however, offers an inexpensive process that gets nanotubes to obediently line themselves up -- that is, self-assemble -- in neat rows, more like ducks.
A broad range of emerging electronic and materials technologies take advantage of the unique physical, optical and electrical properties of carbon nanotubes, but most of them—nanoscale conductors or “nanowires,” for instance—are predicated on the ability to efficiently line the nanotubes up in some organized arrangement. Unfortunately, just mixed in a solvent, the nanotubes will clump together in a black goo.
They can be coated with another molecule to prevent clumping—DNA is sometimes used—but spread the mixture out and dry it and you get a random, tangled mat of nanotubes. There have been a variety of mechanical approaches to orienting carbon nanotubes on a surface (see, for example, “Stretching Exercises Shed New Light on Nanotubes,” www.physorg.com/news95617271.html), but a more elegant and attractive solution would be to get them to do it themselves—self assembly.
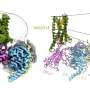
NIST researchers studying better ways to sort and purify carbon nanotubes to prepare standard samples of the material were using a bile acid** to coat the nanotubes to prevent clumping. “Bile acids,” says NIST research chemist Erik Hobbie, “are biological surfactants, and like most surfactants they have a part that likes water and a part that doesn’t. This is a slightly complex surfactant because instead of having a head and a tail, the usual geometry, it has two faces, one that likes water and one that doesn’t.” Mixed in water, such hydrophobic/hydrophilic molecules normally want to group together in hollow spheres with their hydrophobic “tails” sheltered on the inside, Hobbie explains, but the two-faced geometry of this bile acid makes it form hollow rod shapes instead. Conveniently, the hollow rods can house the rod-shaped nanotubes.
As it turns out, there’s a bonus. Over the course of about a day, the bile acid shells cause the nanotubes to begin lining up, end to end, in long strands, and then the strands begin to join together in twisted filaments, like a length of twisted copper wire. The discovery is a long way from a perfect solution for ordering nanotubes, Hobbie cautions, and a lot of development remains to be done. For one thing, ideally, the bile acid shells would be removed after the nanotubes are in their ordered positions, but this has proven difficult. And the surfactant is toxic to living cells, which precludes most biomedical applications unless it is removed.
On the other hand, he says, it already is an easy and extremely inexpensive technique for researchers interested in studying, for example, optical properties of carbon nanotubes. “It gives a recipe for how to create ordered, aligned arrangements of individual carbon nanotubes. You don’t need to use any external magnetic or electrical fields, and you don’t need to dry the tubes out in a polymer and heat it up and stretch it. You can get fairly significant regions of very nice alignment just spontaneously through this self assembly.”
Notes:
* E.K. Hobbie, J.A. Fagan, M.L. Becker, S.D. Hudson, N. Fakhri and M. Pasquali. Self-assembly of ordered nanowires in biological suspensions of single-wall carbon nanotubes. ACS Nano, published online Dec. 16, 2008.
** Sodium deoxycholate.
Source: National Institute of Standards and Technology