New method can capture catalysis, one molecule at a time
(PhysOrg.com) -- Cornell researchers have developed an ingenious microscopic method to observe the behavior of single nanoparticles of a catalyst, down to the resolution of single catalytic events.
Their observations have shown that not all nanoparticles in a batch are created equal: Some carry out their reactions in different ways than others. The researchers have also directly observed that every nanoparticle changes the speed of its catalytic reaction over time, and they have measured the time scale.
There is intense interest in nanocatalysts for such applications as fuel cells and pollutant removal, because nanoparticles provide a larger surface area to speed reactions, and in some cases, materials that are not catalytic in bulk become so at the nanoscale.
"Understanding the fundamental principles that govern the catalyst activity can help us to design new catalysts," said Peng Chen, Cornell assistant professor of chemistry and chemical biology. "Nanoparticles are dynamic entities. Maybe we can think about designing smart catalysts that can adapt to different conditions."
The research by Chen and colleagues is described in the online edition of the journal Nature Materials and will appear in a forthcoming print edition.
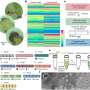
The researchers immobilized spherical gold nanoparticles about 6 nanometers in diameter on a glass surface and flowed a solution of a dye over them. The gold catalyst changes molecules of the dye into a new fluorescent form. Using a microscope that focuses on a very thin plane, the researchers made a "movie" with one frame every 30 milliseconds.
A dye molecule briefly binds to the surface of the gold, where an oxygen atom is removed. The new molecule fluoresces, and a blip of light appears in the microscope image, remaining until the molecule releases from the catalyst. The researchers were able to isolate the blips from individual nanoparticles and identify single catalytic events.
They saw two slightly different reaction patterns: On some nanoparticles the dye molecule binds to the surface, is changed and then releases. On others, after the change the molecule moves to a new position before it releases. And on some nanoparticles, both types of reaction occur. The nanoparticles, Chen explained, are not perfectly spherical, and different parts of the gold crystal are exposed at different places on the surface; this may account for the different reaction patterns.
Reactions at the same sites also varied in their timing. The time a fluorescent molecule remains at a given site might be short, then longer, then short again. The explanation, the researchers said, is that the catalytic reaction also causes a restructuring of the surface of the gold, and this causes the subsequent reactions to take place faster or slower. Later, the gold surface recovers to its original structure, and the reaction returns to its original timing.
"For the first time, we could provide a quantitation on the restructuring timescales at tens to hundreds of seconds," the researchers said.
Provided by Cornell University