Scientists 'see' how HIV matures into an infection
After improving the sensitivity of nuclear magnetic resonance (NMR), researchers at the University of Missouri actually watched the HIV-1 protease mature from an inactive form into an active infection. This process has never been directly visualized before. The findings appear today in the journal Nature.
"We actually saw the process occur," said Chun Tang, assistant professor of biochemistry in the MU School of Medicine. "This is something that has never been done before. We now understand more about the maturation process. We hope this will be a stepping stone to intervening before the infection progresses."
The HIV-1 protease is responsible for releasing the essential building blocks of an infective
HIV-1 viral particle, the culprit of AIDS. The HIV-1 protease is one of the primary targets of therapeutic treatment. However, the viral enzyme is constantly mutating in an effort to gain drug resistance.
"HIV-1 protease is not an active enzyme when it is first expressed in cells. It has to be activated to do its job," Tang said. "What we were able to see is how it self-activates from an immature form when the virus is not infective into a mature form when the virus gains infectivity."
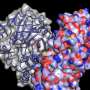
Tang and his colleagues used a novel NMR method called paramagnetic resonance relaxation enhancement and were able to see the temporary joining of two halves of HIV-1 protease precursor, something that had not been accessible before using conventional techniques.
The researchers discovered that the 'tail,' or the flanking amino acid residues, of the HIV-1 protease precursor go through a temporarily formed tunnel where the tail is cut off. At this point, the protease becomes active, the maturation process proceeds, and the virus becomes infective.
"The more we understand about the virus, especially about the maturation into infection, the more we can do to identify novel therapeutics," Tang said.
Citation: The study, "Visualizing transient events in amino-terminal autoprocessing of HIV-1 protease," was published today in the journal Nature.
Source: University of Missouri-Columbia