New graphene-based material clarifies graphite oxide chemistry

A new "graphene-based" material that helps solve the structure of graphite oxide and could lead to other potential discoveries of the one-atom thick substance called graphene, which has applications in nanoelectronics, energy storage and production, and transportation such as airplanes and cars has been created by researchers at The University of Texas at Austin.
To get an idea of the nanomaterial graphene, imagine a lightweight material having the strongest chemical bond in nature and, thus, exceptional mechanical properties. In addition it conducts heat better than any other material and has charge carriers moving through it at a significant fraction of the speed of light. Just an atom thick, graphene consists of a "chickenwire" (or honeycomb) bonding arrangement of carbon atoms – also known as a single layer of graphite.
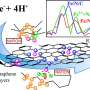
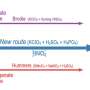
Mechanical Engineering Professor Rod Ruoff and his co-authors have, for the first time, prepared carbon-13 labeled graphite. They did this by first making graphite that had every "normal" carbon atom having the isotope carbon-12, which is magnetically inactive, replaced with carbon-13, which is magnetically active. They then converted that to carbon-13 labeled graphite oxide and used solid-state nuclear magnetic resonance to discern the detailed chemical structure of graphite oxide.
The work by Ruoff's team will appear in the Sept. 26 issue of the journal Science.
"As a result of our work published in Science, it will now be possible for scientists and engineers to create different types of graphene (by using carbon-13 labeled graphene as the starting material and doing further chemistry to it) and to study such graphene-based materials with solid-state nuclear magnetic resonance to obtain their detailed chemical structure," Ruoff says. "This includes situations such as where the graphene is mixed with a polymer and chemically bonded at critical locations to make remarkable polymer matrix composites; or embedded in glass or ceramic materials; or used in nanoelectronic components; or mixed with an electrolyte to provide superior supercapacitor or battery performance. If we don't know the chemistry in detail, we won't be able to optimize properties."
Graphene-based materials are a focus area of research at the university because they are expected to have applications for ultra-strong yet lightweight materials that could be used in automobiles and airplanes to improve fuel efficiency, the blades of wind turbines for improved generation of electrical power, as critical components in nanoelectronics that could have blazing speeds but very low power consumption, for electrical energy storage in batteries and supercapacitors to enable renewable energy production at a large scale and in transparent conductive films that will be used in solar cells and image display technology. In almost every application, sensitive chemical interactions with surrounding materials will play a central role in understanding and optimizing performance.
Ruoff and his team proved they had made such an isotopically-labeled material from measurements by co-author Frank Stadermann of Washington University in St Louis. Stadermann used a special mass spectrometer typically used for measuring the isotope abundances of various elements that are in micrometeorites that have landed on Earth. Then, 100 percent carbon-13 labeled graphite was converted to 100 percent carbon-13 labeled graphite oxide, also a layered material but with some oxygen atoms attached to the graphene by chemical bonds.
Co-authors Yoshitaka Ishii and Medhat Shaibat of the University of Illinois-Chicago then used solid state nuclear magnetic resonance to help reveal the detailed chemical bonding network in graphite oxide. Ruoff says even though graphite oxide was first synthesized more than150 years ago the distribution of oxygen atoms has been debated even quite recently.
"The ability to control the isotopic labeling between carbon-12 and carbon-13 will lead to many other sorts of studies," says Ruoff, who holds the Cockrell Family Regents Chair in Engineering #7.
He collaborates on other graphene projects with other university scientists and engineers such as Allan MacDonald (Department of Physics and Astronomy), Sanjay Banerjee, Emanuel Tutuc and Bhagawan Sahu (Department of Electrical and Computer Engineering) and Gyeong Hwang (Department of Chemical Engineering), and some of these collaborations include industrial partners such as Texas Instruments, IBM and others.
To learn more about Ruoff's work, visit: bucky-central.me.utexas.edu/ .
Source: University of Texas at Austin