Golden Nanorods for Medical Applications
(PhysOrg.com) -- Gold nanoparticles are under consideration for a number of biomedical applications, such as tumor treatment. A German-American research team at Carnegie Mellon University in Pittsburgh, Hunter College in New York, and the RWTH Aachen has now developed a new method for the production of nanoscopic gold rods. In contrast to previous methods, they have achieved this without the use of cytotoxic additives. As they report in the journal Angewandte Chemie, the synthesis is not carried out in water, but in an ionic liquid, a “liquid salt”.
Cancer cells are relatively temperature-sensitive. This is exploited in treatments involving overheating of parts of the cancer patient’s body. One highly promising method is photoinduced hyperthermia, in which light energy is converted to heat. Gold nanoparticles absorb light very strongly in the near infrared, a spectral region that is barely absorbed by tissue. The absorbed light energy causes the gold particles to vibrate and is dissipated into the surrounding area as heat. The tiny gold particles can be functionalized so that the specifically bind to tumor cells. Thus, only cells that contain gold particles are killed off.
The problem? Ordinary spherical gold particles do not efficiently convert the light energy into heat; only rod-shaped particles will do. Unfortunately, the additives needed to crystallize the rod-shaped particles from aqueous solutions are cytotoxic.
The team headed by Michael R. Bockstaller is now pursuing a new strategy: instead of aqueous solution, they chose to use an ionic liquid as their medium of crystallization. Ionic liquids are “liquid salts”, organic compounds that exist as oppositely charged ions, but in the liquid state. In this way, the researchers have been able to produce gold nanorods without the use of any cytotoxic additives.
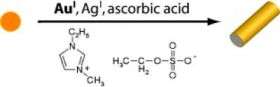
In the first step, seed crystals are produced in the form of tiny spherical gold particles. These crystals are added to a “secondary growth solution” containing monovalent gold ions, silver ions, and the weak reducing agent ascorbic acid. The solvent is an imidazolium-based ionic liquid. In this medium, the crystals don’t continue to grow into spheres; instead they form rods with the round crystallization nuclei as “heads”. The mechanism is presumed to involve the various, energetically inequivalent surfaces of the crystal lattice: the aromatic, nitrogen-containing five-membered rings of the ionic liquid prefer to accumulate at the highly energetic facets of gold surfaces. They thus stabilize crystal shapes that have fewer low-energy facets than the normal spherical equilibrium form. This results in long rods.
Citation: Michael R. Bockstaller, Imidazolium-Based Ionic Liquids as Efficient Shape-Regulating Solvents for the Synthesis of Gold Nanorods, Angewandte Chemie International Edition 2008, 47, No. 40, doi: 10.1002/anie.200802185
Provided by Wiley





















