Turning Waste Material into Ethanol
(PhysOrg.com) -- Say the word “biofuels” and most people think of grain ethanol and biodiesel. But there’s another, older technology called gasification that’s getting a new look from researchers at the U.S. Department of Energy’s Ames Laboratory and Iowa State University. By combining gasification with high-tech nanoscale porous catalysts, they hope to create ethanol from a wide range of biomass, including distiller’s grain left over from ethanol production, corn stover from the field, grass, wood pulp, animal waste, and garbage.
Gasification is a process that turns carbon-based feedstocks under high temperature and pressure in an oxygen-controlled atmosphere into synthesis gas, or syngas. Syngas is made up primarily of carbon monoxide and hydrogen (more than 85 percent by volume) and smaller quantities of carbon dioxide and methane.
It’s basically the same technique that was used to extract the gas from coal that fueled gas light fixtures prior to the advent of the electric light bulb. The advantage of gasification compared to fermentation technologies is that it can be used in a variety of applications, including process heat, electric power generation, and synthesis of commodity chemicals and fuels.
“There was some interest in converting syngas into ethanol during the first oil crisis back in the 70s,” said Ames Lab chemist and Chemical and Biological Science Program Director Victor Lin. “The problem was that catalysis technology at that time didn’t allow selectivity in the byproducts. They could produce ethanol, but you’d also get methane, aldehydes and a number of other undesirable products.”
A catalyst is a material that facilitates and speeds up a chemical reaction without chemically changing the catalyst itself. In studying the chemical reactions in syngas conversion, Lin found that the carbon monoxide molecules that yielded ethanol could be “activated” in the presence of a catalyst with a unique structural feature.
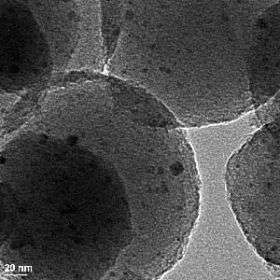
In this transmission electron micrograph of the mesoporous nanospheres, the nano-scale catalyst particles show up as the dark spots. Using particles this small (~ 3nm) increases the overall surface area of the catalyst by roughly 100 times.
“If we can increase this ‘activated’ CO adsorption on the surface of the catalyst, it improves the opportunity for the formation of ethanol molecules,” Lin said. “And if we can increase the amount of surface area for the catalyst, we can increase the amount of ethanol produced.”
Lin’s group looked at using a metal alloy as the catalyst. To increase the surface area, they used nano-scale catalyst particles dispersed widely within the structure of mesoporous nanospheres, tiny sponge-like balls with thousands of channels running through them. The total surface area of these dispersed catalyst nanoparticles is roughly 100 times greater than the surface area you’d get with the same quantity of catalyst material in larger, macro-scale particles.
It is also important to control the chemical makeup of the syngas. Researchers at ISU's Center for Sustainable Environmental Technologies , or CSET, have spent several years developing fluidized bed gasifiers to provide reliable operation and high-quality syngas for applications ranging from replacing natural gas in grain ethanol plants to providing hydrogen for fuel cells.
“Gasification to ethanol has received increasing attention as an attractive approach to reaching the Federal Renewable Fuel Standard of 36 billion gallons of biofuel,” said Robert Brown, CSET director.
“The great thing about using syngas to produce ethanol is that it expands the kinds of materials that can be converted into fuels,” Lin said. “You can use the waste product from the distilling process or any number of other sources of biomass, such as switchgrass or wood pulp. Basically any carbon-based material can be converted into syngas. And once we have syngas, we can turn that into ethanol.”
Provided by Iowa State University





















