Researchers analyze material with 'colossal ionic conductivity'
A new material characterized at the Department of Energy's Oak Ridge National Laboratory could open a pathway toward more efficient fuel cells.
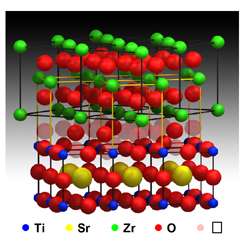
The material, a super-lattice developed by researchers in Spain, improves ionic conductivity near room temperature by a factor of almost 100 million, representing "a colossal increase in ionic conduction properties," said Maria Varela of ORNL's Materials Science and Technology Division, who characterized the material's structure with senior researcher Stephen Pennycook.
The paper, a collaboration between researchers at the Universities of Madrid and at ORNL, was published today in Science.
The analysis was done with ORNL's 300 kilovolt Z-contrast scanning transmission electron microscope, which can achieve aberration-corrected resolutions near 0.6 angstrom, until recently a world record. The direct images show the crystal structure that accounts for the material's conductivity.
"It is amazing," Varela said. "We can see the strained, yet still ordered, interface structure that opens up a wide pathway for ions to be conducted."
Solid oxide fuel cell technology requires ion-conducting materials -- solid electrolytes -- that allow oxygen ions to travel from cathode to anode. However, existing materials have not provided atom-scale voids large enough to easily accommodate the path of a conducted ion, which is much bigger than, for example, an electron.
"The new layered material solves this problem by combining two materials with very different crystal structures. The mismatch triggers a distortion of the atomic arrangement at their interface and creates a pathway through which ions can easily travel," Varela said.
Other fuel cell materials force ions to travel through tight pathways with few spaces for the ions to occupy, slowing their progress. Rather than forcing the ions to jump from hole to hole, the new material has "lots of vacant spaces to be occupied," said Varela, so the ions can travel much more quickly.
Unlike previous fuel cell materials, which have to achieve high temperatures to conduct ions, the new material maintains ionic conductivity near room temperatures. High temperatures have been a major roadblock for developers of fuel cell technology.
The research team with Spain's Universidad Complutense de Madrid and Universidad Politécnica de Madrid produced the material and observed its outstanding conductivity properties, but the structural characteristics that enable the material to conduct ions so well were not known until the material was put under the ultra-high resolution microscopes at ORNL.
Source: Oak Ridge National Laboratory





















