Researchers develop new monomer fluorescent protein for SR imaging
To understand the cell, it is necessary to study its dynamics at high resolution in space and time using techniques that do not adversely affect it. Recently developed superresolution (SR) microscopy breaks the diffraction limit and offers the requisite spatial resolution but usually at the cost of slow imaging speed and excessive damage. Applying reversibly switchable fluorescent proteins (RSFPs) greatly reduces the illumination intensity, thus enabling live-cell SR imaging while using saturated depletion-based SR techniques such as nonlinear structured illumination microscopy (SD NL-SIM) or reversible saturable optical fluorescence transition (RESOLFT) microscopy.
However, one major challenge in live-cell SR is the absence of optimal fluorescent probes. The inherent optical properties of the existing switchable fluorescent proteins Dronpa and rsEGFP—including the small number of switching cycles, low fluorescence signal, and poor contrast—make it difficult to achieve the desired resolution in live-cell SR imaging.
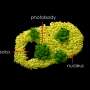
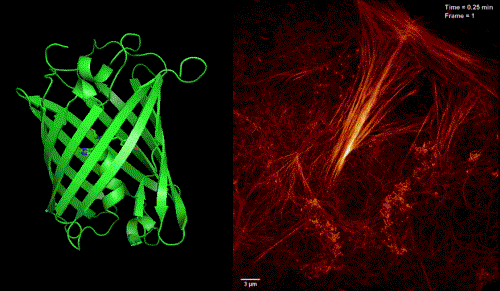
To circumvent the problem, Professor XU Pingyong at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences recently developed a new type of monomer, RSFP Skylan-NS (sky lantern for nonlinear structured illumination).
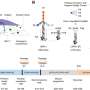
Professor XU, in cooperation with Eric Betzig, a researcher and Nobel laureate at HHIM, and LI Dong, formerly a postdoctoral fellow under Dr. Betzig and now a professor at IBP, applied Skylan-NS to their previously developed SR imaging technique, patterned activation nonlinear SIM (PA NL-SIM).
PA NL-SIM is much more compatible with noninvasive live-cell imaging at a resolution below 100 nm than other SR modalities. This technique uses specific RSFPs, but the properties that strongly influence their suitability for PA NL-SIM have not been enumerated, measured, and compared.
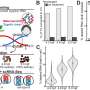
In their study, the researchers performed such a comparison by evaluating the photophysical properties of Skylan-NS against two other RSFPs, i.e., rsEGFP2 and Dronpa, which have been used previously in the SR imaging modalities of RESOLFT and SD NL-SIM, respectively.
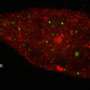
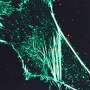
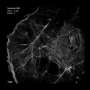
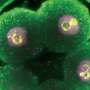
They further demonstrated the superiority of Skylan-NS for PA NL-SIM by comparing the imaging performance of all three RSFPs when applied to PA NL-SIM. For the first time, they achieved low-energy (100 W/cm2), high-sampling speed (sub-second level), high-resolution (~ 60 nm) and long-term (~30 point in time) super-resolution imaging in living cells. Due to its superiority in photostability, cycle numbers and signal-to-noise ratio, Skylan-NS is one of the best fluorescent proteins applicable to live-cell SR imaging.
The study, titled "Highly Photostable, Reversibly Photoswitchable Fluorescent Protein with High Contrast Ratio for Live-cell Superresolution Microscopy," was published online in the journal Proceedings of the National Academy of Sciences on August 23, 2016. The fluorescent protein Skylan-NS enables substantial improvements in the speed, duration, and noninvasiveness of live-cell superresolution microscopy.
More information: Xi Zhang et al. Highly photostable, reversibly photoswitchable fluorescent protein with high contrast ratio for live-cell superresolution microscopy, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1611038113
Journal information: Proceedings of the National Academy of Sciences
Provided by Chinese Academy of Sciences