The science of watching paint dry

New research published today in the journal Physical Review Letters has described a new physical mechanism that separates particles according to their size during the drying of wet coatings. The discovery could help improve the performance of a wide variety of everyday goods, from paint to sunscreen.
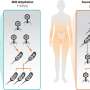
Researchers from the University of Surrey in collaboration with the Université Claude Bernard, Lyon used computer simulation and materials experiments to show how when coatings with different sized particles, such as paints dry, the coating spontaneously forms two layers.
This mechanism can be used to control the properties at the top and bottom of coatings independently, which could help increase performance of coatings across industries as diverse as beauty and pharmaceuticals.
Dr Andrea Fortini, of the University of Surrey and lead author explained:
"When coatings such as paint, ink or even outer layers on tablets are made, they work by spreading a liquid containing solid particles onto a surface, and allowing the liquid to evaporate. This is nothing new, but what is exciting is that we've shown that during evaporation, the small particles push away the larger ones, remaining at the top surface whilst the larger are pushed to bottom. This happens naturally."
Dr Fortini continued, "This type of 'self-layering' in a coating could be very useful. For example, in a sun screen, most of the sunlight-blocking particles could be designed to push their way to the top, leaving particles that can adhere to the skin near the bottom of the coating. Typically the particles used in coatings have sizes that are 1000 times smaller than the width of a human hair so engineering these coatings takes place at a microscopic level. "
The team is continuing to work on such research to understand how to control the width of the layer by changing the type and amount of small particles in the coating and explore their use in industrial products such as paints, inks, and adhesives
Journal information: Physical Review Letters
Provided by University of Surrey