Male mice without any Y chromosome genes can father offspring after assisted reproduction
The Y chromosome is a symbol of maleness, present only in males and encoding genes important for male reproduction. But a new study has shown that live mouse progeny can be generated with assisted reproduction using germ cells from males which do not have any Y chromosome genes. This discovery adds a new light to discussions on Y chromosome gene function and evolution. It supports the hypothesis that Y chromosome genes can be replaced by that encoded on other chromosomes.
Two years ago, the University of Hawaii (UH) team led by Monika A. Ward, Professor at the Institute for Biogenesis Research, John A. Burns School of Medicine, University of Hawai'i, demonstrated that only two genes of the Y chromosome, the testis determinant factor Sry and the spermatogonial proliferation factor Eif2s3y, were needed for male mice to sire offspring with assisted fertilization. Now, the same team, with a collaborating researcher from France, Michael Mitchell (INSERM, Marseille), took a step further and produced males completely devoid of the entire Y chromosome.
In this new study scheduled for online publication in the journal Science on Jan. 29, 2016, Ward and her UH colleagues describe how they generated the "No Y" males, and define the ability of these males to produce gametes and sire offspring.
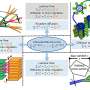
The UH researchers first replaced the Y chromosome gene Sry with its homologue and direct target encoded on chromosome 11, Sox9. In normal situation, Sry activates Sox9, and this initiates a cascade of molecular events that ultimately allow an XY fetus to develop into a male. The researchers used transgenic technology to activate Sox9 in the absence of Sry.
Next, they replaced the second essential Y chromosome gene, Eif2s3y, with its X chromosome encoded homologue, Eif2s3x. Eif2s3y and Eif2s3x belong to the same gene family and are very similar in sequence. The researchers speculated that these two genes may play similar roles, and it is a global dosage of both that matters. They transgenically overexpressed Eif2s3x, increasing dose of the X gene beyond that provided normally by X and Y. Under these conditions, Eif2s3x took over the function of Eif2s3y in initiating spermatogenesis.
Finally, Ward's team replaced Sry and Eif2s3y simultaneously, and created XOSox9,Eif2s3x males that had no Y chromosome DNA. Mice lacking all Y chromosome genes developed testes populated with male germ cells. Round spermatids were harvested and a technique called round spermatid injection (ROSI) was used to successfully fertilize oocytes. When the developed embryos where transferred to female mouse surrogate mothers, live offspring were born.
The offspring derived from the "No Y" males were healthy and lived for normal life span. The daughters and grandsons of the "No Y" males were fertile and capable of reproducing on its own without further technological intervention. Ward's team produced three consecutive generations of "No Y" males using ROSI showing that males lacking Y chromosome genes can be repeatedly propagated with technical assistance.
"Most of the mouse Y chromosome genes are necessary for development of mature sperm and normal fertilization, both in mice and in humans," Ward said. "However, when it comes to assisted reproduction, we have now shown that in the mouse the Y chromosome contribution is not necessary."
The study provides new important insights into Y chromosome gene function and evolution. It supports the existence of functional redundancy between the Y chromosome genes and their homologues encoded on other chromosomes. "This is good news," Ward said, "because it suggests that there are back-up strategies within genomes, which are normally silent but are capable of taking over under certain circumstances. We revealed two of these strategies by genome manipulation. Whether such alternative pathways would ever be activated without human help, for example in response to environmental changes, is unknown. But it is certainly possible and has already happened for two rodent species which lost their Y chromosomes. "
The development of assisted reproduction technologies (ART) allows bypassing various steps of normal fertilization by using immotile, non-viable, or immature gametes. The newest study as well as Ward's preceding report (Science 2014 Jan 3; 343 (6166: 69-72) support that in the mouse ROSI is a successful and efficient form of ART. In humans, ROSI is considered experimental due to concerns regarding the safety of injecting immature germ cells and other technical difficulties. The researchers hope that the success in mouse studies may spark the re-evaluation of human ROSI for its suitability to become an option for overcoming male infertility in the future.
More information: Two genes substitute for the mouse Y chromosome for spermatogenesis and reproduction, DOI: 10.1126/science.aad1795
Journal information: Science
Provided by University of Hawaii at Manoa