June 5, 2015 report
New inorganic aromatic ion
![Solid-state molecular structure of [Nakryptofix-221] [P2N3]. Credit: (c) Science, DOI: 10.1126/science.aab0204 New inorganic aromatic ion](https://scx1.b-cdn.net/csz/news/800a/2015/5571ca3de7bc3.jpg)
(Phys.org)—Aromatic molecules are a staple of organic chemistry. Aromatics, like benzene, have a unique pi-electron character that makes the ring structure exceptionally stable. While aromatics are typically reserved for carbon containing compounds, there have been some cases of inorganic aromatics containing the Group 15 elements. Alexandra Velian and Christopher C. Cummins of MIT report in Science the synthesis and characterization of a novel inorganic aromatic anion, P2N3-.
What makes this new five-atom, six-electron aromatic molecule so interesting is its simple structure and straight-forward synthesis. As Dr. Cummins points out, "Sometimes when a molecule that looks perfectly reasonable does not exist, the reason may be as simple as nobody has yet found a way to make it. If the molecule can then be made, this opens the door to determining its properties and potential applications."
P2N3- was synthesized using a diphosphorus source that comes from anthracene (A). Prior studies by this group determined that upon heating P2A2 would release P2 and two anthracene molecules, providing a ready source of P2, which is itself not sufficiently stable for isolation. For this research, they combined P2A2 with tetrabutylammonium azide in THF and heated for three hours. While characterization confirmed the formation of the target aromatic anion, they were unable to isolate the anion from excess tetrabutylammonium azide and anthracene.
To overcome this, Velian and Cummins performed the same reaction but with sodium kryptofix-221, a commercially available macrocycle that encapsulates sodium ions. This provided an appropriate counter ion for the azide reactant and anion product and also solved the solubility issues that had precluded product isolation.
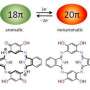
While NMR can provide evidence for product formation, several characterization techniques were needed to confirm that P2N3- is truly an aromatic compound. X-ray diffraction studies showed that P2N3- is a planar ring. NMR studies showed that this symmetry changes in nonpolar solvents, compared to polar solvents, which may be due to the nitrogen atoms partially coordinating to the macrocycle. X-ray studies also showed that the P-P and P-N bond distances are in between the values for single and double bonds and are similar to calculated aromatic values.
Nucleus independent chemical shift, a technique that calculates magnetic shielding within the center of the ring structure, also provided corroborating evidence that P2N3- is likely an aromatic compound. P2N3- was compared to other five-membered, six-electron aromatic rings (e.g., N5-, P5-, C5H5-, and N2S32+) and was found to have comparable magnetic shifts. Additional studies showed that the pi orbitals were largely responsible for magnetic aromaticity.
NMR, IR, and Raman spectroscopy provided further evidence of aromaticity. 31P NMR showed one phosphorus peak at 334 ppm indicating the phosphorus atoms are chemically equivalent. IR and Raman studies with 15N labeling of one of the nitrogen atoms that is bonded to phosphorus indicated the expected red shift compared to unlabeled product. These spectra also showed that the P-P bond did not change compared to the unlabeled anion. Compared to P5- and P2H2, the P-P bond in P2N3- is stronger than that in P5- and weaker than the P-P bond in P2H2.
Velian and Cummins have provided several lines of evidence that their Group 15 anionic ring is likely a novel aromatic molecule. Their synthesis involved the use of a phosphorus-anthracene reactant that, when combined with as source of the azide ion, N3-, in the presence of an organometallic counterion, allowed for a one-step synthesis of the target compound. Furthermore, their work provides an opportunity to study phosphorus-phosphorus pi bonding in a simple planar molecule.
More information: "Synthesis and characterization of P2N3-: An aromatic ion composed of phosphorus and nitrogen" Science, DOI: 10.1126/science.aab0204
ABSTRACT
Aromaticity is predominantly associated with carbon-rich compounds but can also occur in all-inorganic ones. We report the synthesis of the diphosphatriazolate anion, a rare example of a planar aromatic inorganic species. Treatment of azide (N3−) in tetrahydrofuran solution with P2A2 (A = C14H10), a source of P2, produced P2N3−, which we isolated as its [Na-kryptofix-221]+ salt in 22% yield and characterized by single-crystal x-ray diffraction. Salts [Na-kryptofix-221] [P2N3] and [Na-kryptofix-221] [P215NN2] were analyzed by infrared and Raman spectroscopy, 15N and 31P nuclear magnetic resonance spectroscopy, and mass spectrometry. The formation of the P2N3− anion was investigated using density functional theory, and its aromatic character was confirmed by NICS (nucleus-independent chemical shift) and QTAIM (quantum theory of atoms in molecules) methods.
Journal information: Science
© 2015 Phys.org