New material captures carbon dioxide with high capacity

A new provisionally patented technology from a New Mexico State University researcher could revolutionize carbon dioxide capture and have a significant impact on reducing pollution worldwide.
The Intellectual Property and Technology Transfer Office at NMSU's Arrowhead Center is working to protect and commercialize the technology, which was developed by chemical and materials engineering doctoral candidate Nasser Khazeni.
With support from NMSU faculty members Abbas Ghassemi, Reza Foudazi and Jalal Rastegary, Khazeni has developed a special material that can capture carbon dioxide with greater capacity than any technology currently in widespread use for that purpose.
Technology licensing associate Theresa Lombard helped Khazeni obtain a provisional patent for the technology.
"This technology is going to radically impact the world with regard to carbon dioxide released into the atmosphere," Lombard said. "It's exciting."
According to the U.S. Department of Energy, the U.S. generated more than 3.18 billion metric tons of carbon dioxide in 2013, of which two thirds was attributed to power plants alone. In general, about 20 to 30 percent of a power plant's energy is spent on capturing carbon dioxide emissions, at a cost of $70 per metric ton.
"It's expensive and has a negative environmental impact," Lombard said. "This new technology is a solution to both of those problems."
Khazeni's focus is on the post-combustion separation of carbon dioxide – how can it be more efficiently separated out, transported and stored or reused.
He described a chain of cause and effect in which human activity – primarily fossil fuel consumption – leads to increased concentration of greenhouse gases in the atmosphere, leading to global warming and climate change.
"To resolve this issue," Khazeni said, "we need to take one of the links of this chain and mitigate it before it reaches the global warming stage. We're addressing the middle link – capturing carbon dioxide in the atmosphere."
A common method of carbon dioxide capture is absorption, which captures molecules in a liquid medium. Releasing the gas from the liquid medium for storage or use for things like fueling algae growth is very costly and inefficient.
Through another method – adsorption – the carbon dioxide physically bonds to a microporous, sponge-like solid surface. Releasing the carbon dioxide from the solid surface is much more cost- and energy-efficient than releasing it from a liquid medium.
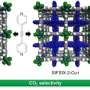
Khazeni's research focuses on solid adsorbents, which capture carbon dioxide and store it for transport or storage. A hybrid metal and organic structure called a zeolitic imidazolate framework adsorbs carbon dioxide molecules to its structure of metal ions and organic linkers.
Through his research on these zeolitic imidazolate frameworks, or ZIFs, Khazeni has synthesized a new subclass of ZIF that incorporates a ring carbonyl group in its organic structure, giving it a vastly greater affinity and selectivity for separating and adsorbing carbon dioxide and a more chemically and thermally stable structure.
In a simulation study Khazeni conducted, the new ZIF structure adsorbed more than 100 times more carbon dioxide than other similar structures. With negligible difference in adsorption of other gases like nitrogen and hydrogen, they can also separate carbon dioxide from gas mixtures more selectively.
Seeing that vastly increased selectivity and capture capacity – and its potential applications – led Khazeni to seek to protect the idea with a provisional patent, which he obtained in October with the help of Lombard and the Intellectual Property and Technology Transfer Office.
Protecting the idea is the first step, he said, before he could approach a company about the potential application of the research.
"This type of research was very susceptible; just talking about this type of linker that nobody has tried is enough for a good chemist to go out and try it," Khazeni said. "There's competition, so we needed to protect it. Now that it's protected, I can write a proposal and go to companies about commercial applications of the idea."
Lombard said NMSU's intellectual property policy dictates that 50 percent of the proceeds of inventions that are created and licensed at NMSU go back to the inventor and 50 percent goes back to NMSU. The portion that goes to NMSU is split into thirds: one-third goes to the inventor's department, one-third to the inventor's college and the final third goes to the Office of the Vice President for Research for development of additional research opportunities for technologies that could be commercialized.
Lombard said the next step is licensing the technology, and conversations are beginning with major energy industry contacts to do that. The global carbon capture, use and storage market is expected to reach roughly $6.8 billion by 2019, and experience more than 27 percent growth from 2013 to 2019, according to the Department of Energy.
"This technology is very scalable and the market is ready for it," Lombard said. "It's going to change the world, with regard to carbon dioxide capture."
Provided by New Mexico State University