Nanoparticles replace needle and thread
(Phys.org) —Stopping bleeding, closing wounds, repairing organs—these are everyday challenges in medical and surgical practice. In the journal Angewandte Chemie, French researchers have now introduced a new method that employs gluing by aqueous nanoparticle solutions to effectively control bleeding and repair tissues. In animal tests, their approach proved easy to apply, rapid and efficient even in situations when conventional methods are traumatic or fail.
Sutures and staples are efficient tools for use in surgery and treating wounds. However, the usefulness of these methods can be limited in inaccessible parts of the body or in minimally invasive surgeries. In addition, stitching damages soft tissues such as liver, spleen, kidney, or lung. A good adhesive could be a useful alternative. The problem is that the adhesion must take place in a wet environment and that the repaired area is immediately put under strain. Previous adhesive technologies have had problems, including insufficient strength, inflammation due to toxic substances, or complicated implementation because a chemical polymerization or cross-linking reaction must be carried out in a controlled manner.
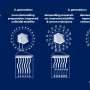
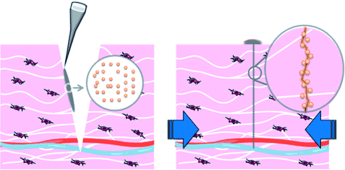
A team headed by Ludwik Leibler at the Laboratoire Matière Molle et Chimie (CNRS/ESPCI Paris Tech) and Didier Letourneur at the Laboratoire Recherche Vasculaire Translationnelle (INSERM/Université Paris Diderot) has now successfully tested a completely novel approach for adhering living tissue: they simply apply droplets of a nanoparticle solution to the wound and press it closed for about a minute. The principle behind is stunningly simple: the nanoparticles spread out across the surface and bind to the tissue's molecular network by attracting interactions. Because there are a very large number of nanoparticles present, millions of bonds firmly bind the two surfaces together. No chemical reaction is needed. The researchers used silicon dioxide and iron oxide nanoparticles for their experiments.
In contrast to conventional wound adhesives, this results in no artificial barrier; it produces direct contact between the two edges of the wound. Because the nanoparticles are so small, they do not appreciably impact the wound healing process. Applied to deep skin wounds the method is easily usable and leads to remarkably aesthetic healing. In addition, it is possible to correct the positioning of the tissue edges relative to each other without opening the wound closure.
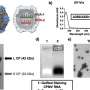
Aqueous solutions of nanoparticles have been also shown to be able to repair rapidly and efficiently in hemorrhagic conditions liver wounds for which sutures are traumatic and not practical. Either a wound was closed and wound edges were glued by nanoparticles or, in the case of liver resections, bleeding was quickly stopped by gluing a polymer strip using a nanoparticle solution.
In addition, the researchers were able to attach a biodegradable membrane to a beating rat heart. This opens new perspectives: it may be possible to attach medical devices for delivering drugs, supporting damaged tissue, as well as matrices for tissue growth.
More information: Ludwik Leibler. "Organ Repair, Hemostasis, and In Vivo Bonding of Medical Devices by Aqueous Solutions of Nanoparticles." Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201401043
Journal information: Angewandte Chemie International Edition
Provided by Angewandte Chemie