Carbon nanotubes grow in combustion flames
Professor Stephan Irle of the Institute of Transformative Bio-Molecules (WPI-ITbM) at Nagoya University and co-workers at Kyoto University, Oak Ridge National Lab (ORNL), and Chinese research institutions have revealed through theoretical simulations that the molecular mechanism of carbon nanotube (CNT) growth and hydrocarbon combustion actually share many similarities.
In studies using acetylene molecules (ethyne; C2H2, a molecule containing a triple bond between two carbon atoms) as feedstock, the ethynyl radical (C2H), a highly reactive molecular intermediate was found to play an important role in both processes forming CNTs and soot, which are two distinctively different structures.
The study published online on January 24, 2014 in Carbon, is expected to lead to identification of new ways to control the growth of CNTs and to increase the understanding of fuel combustion processes.
CNTs are molecules with a cylindrical nanostructure (nano = 10E-9 m or 1 / 1,000,000,000 m). Arising from their unique physical and chemical properties, CNTs have found technological applications in the fields of electronics, optics and materials science.
CNTs can be synthesized by a method called chemical vapor deposition, where hydrocarbon vapor molecules are deposited on transition metal catalysts under a flow of non-reactive gas at high temperatures.
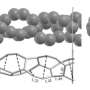
Current issues with this method are that the CNTs are usually produced as mixtures of nanotubes with various diameters and different sidewall structures. Theoretical simulations coordinated by Professor Irle have looked into the molecular mechanisms of CNT growth using acetylene molecules as feedstock (Figure 1). The outcome of their research provides insight into identifying new parameters that can be varied to improve the control over product distributions in the synthesis of CNTs.
High level theoretical calculations using quantum chemical molecular dynamics were performed to study the early stages of CNT growth from acetylene molecules on small iron (Fe38) clusters. Previous mechanistic studies have postulated complete breakdown of hydrocarbon source gases to atomic carbon before CNT growth.
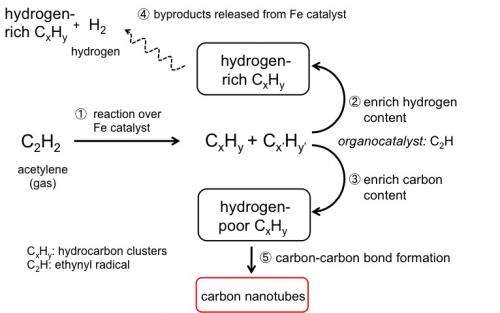
"Our simulations have shown that acetylene oligomerization and cross-linking reactions between hydrocarbon chains occur as major reaction pathways in CNT growth, along with decomposition to atomic carbon" says Professor Stephan Irle, who led the research, "this follows hydrogen-abstraction acetylene addition (HACA)-like mechanisms that are commonly observed in combustion processes" he continues.
Combustion processes are known to proceed by the hydrogen-abstraction acetylene addition (HACA)-like mechanism. Initiation of the mechanism begins with hydrogen atom abstraction from a precursor molecule followed by acetylene addition, and the repetitive cycle leads to formation of ring-structured polycylic aromatic carbons (PAHs).
In this process, the highly reactive ethynyl radical (C2H) is continually being regenerated, extending the rings of PAHs and eventually forming soot. The same key reactive intermediate is observed in CNT growth and acts as an organocatalyst (a catalyst based on an organic molecule) facilitating hydrogen transfer reactions across growing hydrocarbon clusters. The simulations identify an intriguing bifurcation process by which hydrogen-rich hydrocarbon species enrich hydrogen content creating non-CNT byproducts, and hydrogen-deficient hydrocarbon species enrich carbon content leading to CNT growth (Figure 2).
"We started this type of research from 2000, and long simulation time has been a great challenge to conduct full simulations across all participating molecules, due to the relatively high strength of the carbon-hydrogen bond. By establishing and using a fast method of calculation, we were able to successfully incorporate hydrogen in our calculations for the first time, which led to this new understanding revealing the similarity between CNT growth and hydrocarbon combustion processes. This finding is very intriguing in the sense that these processes were long considered to proceed by completely different mechanisms" elaborates Professor Irle.
Results of these simulations illustrate the importance in the role of carbon chemical bonding and molecular transformations in CNT growth. Professor Irle explains, "Our simulations suggest new parameters, such as tuning hydrogen content to improve the control of CNT growth and soot formation. We wish to develop new methods to speed up techniques that will convince experimentalists and establish further tools to explore new possibilities that will contribute to the understanding of these important processes."
More information: "Quantum chemical simulations reveal acetylene-based growth mechanisms in the chemical vapor deposition synthesis of carbon nanotubes" by Ying Wang, Xingfa Gao, Hu-Jun Qian, Yasuhito Ohta, Xiaona Wu, Gyula Eres, Keiji Morokuma, Stephan Irle is published online on January 24, 2014 in Carbon. DOI: 10.1016/j.carbon.2014.01.020
Journal information: Carbon
Provided by Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya