A safer recipe for primordial soup
Researchers have published a simpler, safer method for conducting Miller-Urey origin of life experiments—which may still yield new insight about how life began on Earth.
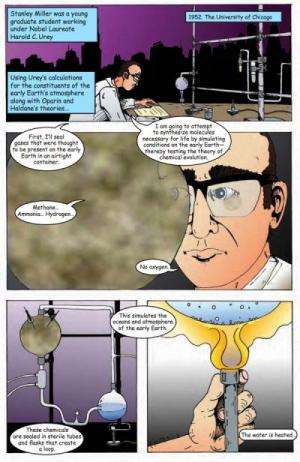
Back in 1953, Stanley Miller, working at the University of Chicago with Harold Urey, showed how easily one could cook up life's building blocks by simulating the conditions on early Earth.
The famous recipe went as follow:
- Boil some water to mimic evaporation of the early ocean.
- Add a few gases thought to be present in the early atmosphere.
- Apply a jolt of electricity to simulate lightning.
- Let run for a few days—and you're left with a brownish soup of amino acids, the building blocks for everything alive on Earth.
But while the success of the Miller-Urey experiment kicked off an entire field of research, Miller had one basic piece of advice for anyone who'd want to try it out: "Don't do it."
"Stanley was always afraid it might lead to a disaster," explains Jeffrey Bada, who was a student of Miller in the 1960s. "If you were not careful to let all the atmospheric air out, the setup could explode. So unless they were highly trained, he'd always advise people against repeating the experiment."
But now a team including scientists from the Georgia Institute of Technology, NASA (including Dr. Bada), and the Tokyo Institute of Technology have recreated a simpler and safer way of conducting Miller-Urey type experiments.
Along with written instructions, the new version was published this month in a step-by-step video format in the Journal of Visualized Experiment.
"In addition to being simpler and safer, the new configuration provides a better representation of Earth's early condition," says Eric Parker, a graduate student at GIT and a lead researcher for the study.
For instance, Miller's original mixture included methane, ammonia, and hydrogen. The new protocol uses nitrogen instead of hydrogen, which we now know is a more accurate representation of the early Earth atmosphere. The likelihood of the experiment exploding is also greatly reduced, since hydrogen is very ignitable in the presence of an electric discharge.
Also, Miller would spark a charge of 60,000 V through the mixture continuously for a week. In the new procedure the charge is set to 30,000 V, and is turned on and off cyclically to mimic the intermittent nature of lightening more accurately.
"There's still a risk since methane is present and also ignitable. But we spell out the inherent risks—and how to avoid them—in clear detail in the new protocol," Parker says.
What's Next?
The researchers hope the new protocol will generate even more interest in the field. "There's still a lot to learn from these experiments," says Bada. "We're barely scratching the surface."
"Some people feel that synthesis of simple compounds has already been done, and so why continue?" he adds. "But I think that's a misrepresentation of the importance of this experiment."
"The next step is figuring out how to go from small molecules (like amino acids) to larger ones (like peptides)—things that have a greater biological functionality," explains Parker. "That's where the next phase of research is kicking in."
What's more, while we know a lot about what's happening in the water phase—where the amino acids are synthesized, we don't know as much about the chemistry going on in the gas phase, explains Bada. "That's an area ripe for research," he says. "From that we can still learn a lot about the chemistry of the early Earth atmosphere, and perhaps even about the atmosphere of other planets."
More information: See issue 1 of "Astrobiology: The story of our search for life in the Universe" here: www.astrobio.net/index.php?option=com_content&id=7
Provided by Astrobio.net



















