July 12, 2013 report
Researchers discover simple coating technique using tannic acid and iron
(Phys.org) —A team of chemical researchers at the University of Melbourne in Australia has discovered a simple coating technique that uses nothing but tannic acid and iron ions. In their paper published in the journal Science, the team describes how they added tannic acid and iron ions to a water solution which resulted in the spontaneous creation of a thin film. The film, the group report, coats other materials that are put into the mix without the need for coaxing.
Tannic acid is one of a number of phenolics—a family of self-assembling biological materials. Others include lignin, found in wood and melanin, found in skin. Tannic acid is of course, found in wine, and has historically been used to tan animal hides. In this new effort, the researchers looked to make use of the material's natural ability to coat surfaces.
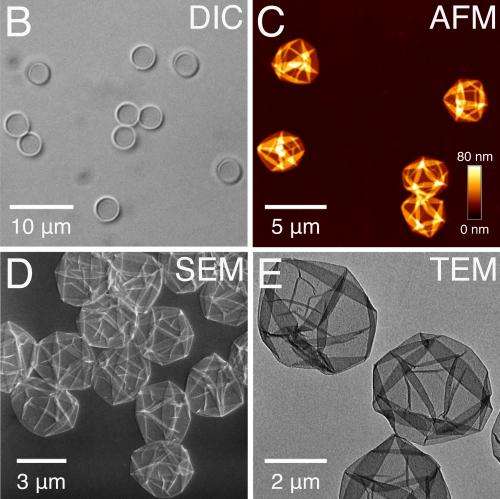
When tannic acid is placed in water it tends to coat anything else that is placed in the water, metals, for example, plastics, or even viruses. Seeking to take advantage of that feature, the researchers added iron ions to the mix, and found that the ions stuck to the acid molecules, resulting in the creation of a thin film. Next, the team added other materials to the mix to serve as various substrates and found that the acid-iron films would spontaneously coat each of them. For small particles, the result was the creation of capsules. Perhaps even more importantly, the team found that by altering the pH level of the solution in which the coating resides, the coating would disassociate itself. Other techniques have been developed to create coatings, of course, but none are as simple (just one step) or inexpensive.
This new way to create a coating is important as it contributes to ongoing research looking into ways to create and manufacture soft matter for use in medical and life science applications. It's not difficult to imagine capsules based on those developed by the team in Australia that hold medicine. Such capsules would release their payload only when they arrive at a part of the body that has the right pH level, allowing for targeted treatment.
More information: One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering, Science 12 July 2013: Vol. 341 no. 6142 pp. 154-157 DOI: 10.1126/science.1237265
ABSTRACT
The development of facile and versatile strategies for thin-film and particle engineering is of immense scientific interest. However, few methods can conformally coat substrates of different composition, size, shape, and structure. We report the one-step coating of various interfaces using coordination complexes of natural polyphenols and Fe(III) ions. Film formation is initiated by the adsorption of the polyphenol and directed by pH-dependent, multivalent coordination bonding. Aqueous deposition is performed on a range of planar as well as inorganic, organic, and biological particle templates, demonstrating an extremely rapid technique for producing structurally diverse, thin films and capsules that can disassemble. The ease, low cost, and scalability of the assembly process, combined with pH responsiveness and negligible cytotoxicity, makes these films potential candidates for biomedical and environmental applications.
Journal information: Science
© 2013 Phys.org