May 27, 2013 feature
Of grains and graphite: Simulating interstellar hydrogen formation
(Phys.org) —The process of molecular hydrogen formation is a key factor in astrophysics – specifically in the physics and chemistry of interstellar clouds. An electrically neutral atom containing a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force, hydrogen is the lightest element and, in its monatomic (unbound single atom) form, known as H1, is the most abundant chemical substance, constituting roughly 75% of the universe's baryonic mass (that is, excluding so-called dark matter and dark energy). Hydrogen formed at an early stage of the universe during expansion, when the temperature dropped enough to reduce the rate of ionization processes and triggered the plasma-neutral phase transition of the primordial gas. This, in turn, decoupled matter from radiation and led to the appearance of the cosmic background radiation.
That being said, monatomic (or, alternatively, atomic) hydrogen atoms are rare, with hydrogen preferring to combine with other atoms in compounds, or with itself to form ordinary (diatomic or molecular) hydrogen gas, or H2. In the interstellar medium (ISM) – which comprises the matter (including gas and dust) that exists in the space between a galaxy's star systems – H2 plays many vital roles, including serving as a chemical precursor for more complex, shielding the clouds from the ambient radiation field, and acting as primary cooling agent during the gravitational collapse that basic to star formation. Moreover, the presence of H2 in the early universe led to the emergence of the first stars and induced galaxy formation.
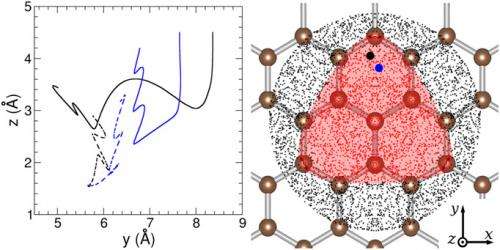
Recently, scientists at Università degli Studi di Milano and Istituto di Scienze e Tecnologie Molecolari, both in Milan, Italy, studied direct (Eley–Rideal, or ER) recombination-based formation – a surface reaction mechanism, proposed in 1938 by D. D. Eley and E. K. Rideal, in which only one of the molecules adsorbs and the other one reacts with it directly from the gas phase, without adsorbing – using ab initio molecular dynamics, or AIMD. (Ab initio techniques employ basic and established laws of nature without additional assumptions or special models.) The researchers found that ER is the dominant reaction at energies consonant with the interstellar medium if so-called facile sticking at edges, point defects, and other dust grain surface sites is possible as the scientists posit. At the same time, the scientists posit that their findings hold promise in research focused on hydrogen-graphite interaction in a range of diverse fields, including hydrogen storage, nuclear fusion, graphene technology, and interstellar chemistry.
Prof. Rocco Martinazzo discussed the research he, Dr. Simone Casolo and Prof. Gian Franco Tantardini conducted, as well as the challenges they faced, with Phys.org. "The ab initio approach in molecular dynamics is a relatively new entry in computational chemistry/physics," Martinazzo tells Phys.org, "and it's now possible thanks to the huge increase of computing power over the past few years." Nevertheless, he adds, it remains highly demanding for quantitative studies where precise statistics are required, meaning that considerable effort is required to find a good compromise between accuracy and computational cost.
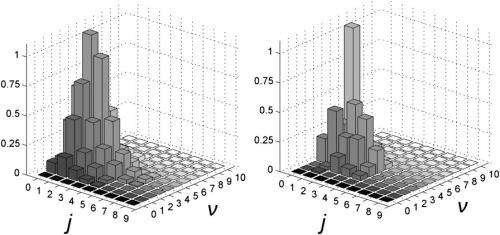
The scientists' molecular dynamics simulation Included lattice dynamics, surface corrugation and competing H-dimers formation processes – an approach usually performed ad hoc, Martinazzo notes, by building model potentials on the basis of a number of reasonable assumptions and fitting them to experimental and/or theoretical data available for specific atomic configurations. "However," he continues, "the procedure is often too involved, and it is not guaranteed to result in a realistic interaction potential for the processes of interest. This is the case with graphite," he illustrates, "where considerable effort has been put in the last few years to devise reliable models able to describe dimer formation, and to include the lattice dynamics in the energy balance of the reaction." More specifically, the first process competes with H2 formation, possibly altering its outcome if not taken into account – but the lattice dynamics determines the correct amount of energy which stored in the hydrogen-hydrogen bond of the newly-formed molecule.
In addressing these challenges, Martinazzo and his colleagues leveraged what they had learned from previous experience – namely, that quantum effects were important at extremely low energy only, and that many of the problems afflicting present results could be tackled with a classical approach. "However," he adds, "we realized though that there was no chance to build a reasonable potential model suited for our problem and decided to adopt an ab initio molecular dynamics approach."
AIMD is the method of choice in these situations since it solves the problem of building a model potential by computing the forces required by the dynamics itself on the fly, and does so by explicitly considering the electrons which generate these forces. "Because of this," Martinazzo points out, "AIMD can be considered an unbiased approach whose accuracy is solely determined by the quality of the level of electronic structure chosen, provided that a classical approach for the atom dynamics is adequate. That being said, I'm sure that thanks to ever-increasing computing power, it will soon become the method of choice for tackling challenging problems at an atomic scale."
In addition, says Martinazzo, the researchers finally established with reasonable accuracy the efficiency of the Eley-Rideal reaction mechanism, and provided thermal rate constants which can be directly used in modeling the chemical evolution of interstellar clouds. "It's actual role in forming hydrogen in space – particularly in those regions which are too hot for the more common Langmuir-Hinshelwood mechanism to occur – remains yet to be established," he acknowledges, "and much depends on the composition and morphology of the dust grain, which something that's hard to assess at present."
Regarding the possibility of facile sticking at special dust grain surface sites, Martinazzo says that they used a very crude model to show that facile sticking may provide a robust mechanism operating under different conditions, but adds that they hope that astrochemists will utilize main results to improve models with accurate microscopic information. "In turn," he adds, "on comparing with observations, this could help deepening our understanding of the nature of dust grains and the role they play in the chemistry of the interstellar medium."
In term of next steps, the scientists are currently investigating how hydrogen atoms stick on graphite. "As suggested in our paper," notes Martinazzo, "the focus is on defective sites where facile sticking may occur – locations where classical dynamics and, fortunately, AIMD work well. However," he adds, "we're also addressing sticking on the basal plane of graphite, in conditions where it can only occur thanks to the quantum nature of matter and its ability to tunnel through classically forbidden regions – a simple but rather challenging problem awaiting definite answers."
Martinazzo also describes other areas of research that might benefit from findings:
- Hydrogen-graphite interactions, which because of their many interesting aspects have long been studied in diverse fields
- In thermonuclear fusion reactors, such as JET and ITER, graphitic compounds are among the plasma facing materials which make up the so-called box which holds the sun – and In the cold plasma region hydrogen is neutralized and impacts on the surface where sticks and eventually reacts
- In the same context, at least in principle, hydrogen formation on graphite may be used for the negative ion injectors which heat up the plasma, since they deliver vibrationally hot molecules that can be readily dissociated by electron impacts, thereby producing negatively charged H atoms
- In graphene technology, hydrogen atoms represent a powerful tool to modify its electronic transport properties, eventually opening a band gap in a controlled way and make graphene suitable for logic applications
"In all these cases," Martinazzo concludes, "knowing details of the behavior of hydrogen atoms on graphene and graphite may help finding the most appropriate operational conditions for achieving specific goals or limiting undesired effects."
More information: Insights into H2 formation in space from ab initio molecular dynamics. PNAS Published online before print April 9, 2013, doi:10.1073/pnas.1301433110
Journal information: Proceedings of the National Academy of Sciences
© 2013 Phys.org. All rights reserved.