Engineers craft new material for high-performing 'supercapacitors'
Taking a significant step toward improving the power delivery of systems ranging from urban electrical grids to regenerative braking in hybrid vehicles, researchers at the UCLA Henry Samueli School of Engineering and Applied Science have synthesized a material that shows high capability for both the rapid storage and release of energy.
In a paper published April 14 in the peer-reviewed journal Nature Materials, a team led by professor of materials science and engineering Bruce Dunn defines the characteristics of a synthesized form of niobium oxide—a compound based on an element used in stainless steel—with a great facility for storing energy. The material would be used in a "supercapacitor," a device that combines the high storage capacity of lithium ion batteries and the rapid energy-delivery ability of common capacitors.
UCLA researchers said the development could lead to extremely rapid charging of devices, ranging in applications from mobile electronics to industrial equipment. For example, supercapacitors are currently used in energy-capture systems that help power loading cranes at ports, reducing the use of hydrocarbon fuels such as diesel.
"With this work, we are blurring the lines between what is a battery and what is a supercapacitor," said Veronica Augustyn, a graduate student in materials science at UCLA and lead author of the paper. "The discovery takes the disadvantages of capacitors and the disadvantages of batteries and does away with them."
Batteries effectively store energy but do not deliver power efficiently because the charged carriers, or ions, move slowly through the solid battery material. Capacitors, which store energy at the surface of a material, generally have low storage capabilities.
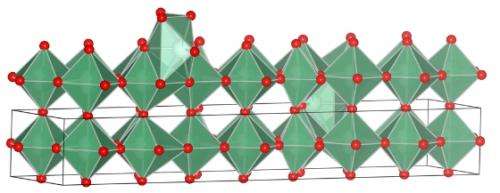
Researchers on Dunn's team synthesized a type of niobium oxide that demonstrates substantial storage capacity through "intercalation pseudocapacitance," in which ions are deposited into the bulk of the niobium oxide in the same way grains of sand can be deposited between pebbles.
As a result, electrodes as much as 40 microns thick—about the same width as many commercial battery components—can quickly store and deliver energy on the same time scales as electrodes more than 100 times thinner.
Dunn emphasizes that although the electrodes are an important first step, "further engineering at the nanoscale and beyond will be necessary to achieve practical devices with high energy density that can charge in under a minute."
Journal information: Nature Materials
Provided by University of California, Los Angeles