Observing how EGFR clusters malfunction in cancer cells

For scientists to improve cancer treatments with targeted therapeutic drugs, they need to be able to see proteins prevalent in the cancer cells. This has been an impossible, until now. Thanks to a new microscopy technique, University of Akron researcher Dr. Adam Smith, assistant professor of chemistry, has observed how clusters of epidermal growth factor receptor (EGFR)—a protein abundant in lung and colon cancers, glioblastoma and others—malfunctions in cancer cells.
"We can directly observe protein clusters, in a living cell membrane, that are invisible to traditional methods. This opens up the possibility to directly measure the effect of drugs on the target proteins," Smith says.
Smith's work lies at the heart of current-day cancer research, which focuses on developing targeted drugs that kill cancer cells without the collateral damage associated with traditional treatments like chemotherapy.
Specifically, Smith used a cutting-edge photon-counting technique, which enables scientists to measure the cluster size of EGFR proteins. The technique represents a significant advancement from studying the cultures with a traditional microscope, which cannot visually capture objects as small as the EGFR clusters, according to Smith, a lead author of "Conformational Coupling across the Plasma Membrane in Activation of the EGF Receptor," published in the Jan. 31 issue of the journal Cell, which highlights the technique.
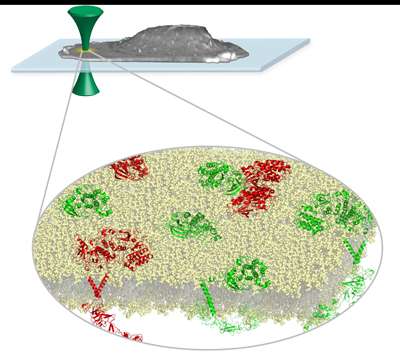
"Another difficulty with studying EGFR is that it's located in the cell membrane, which can be thought of as a fence line that defines the cell boundary, but in reality it is more like an untamed hedge row," says Smith, explaining how the new laser-based microscope technique overcomes that obstacle and allows scientists to study, in real time, how EGFR works in healthy cells and also how it malfunctions in cancer cells.
Smith's subsequent work studying the interaction of drugs with the targeted EGFR "will significantly improve drug discovery, which too often relies on indirect measure of efficacy," he says.
More information: www.cell.com/abstract/S0092-8674(12)01554-1
Journal information: Cell
Provided by University of Akron
















