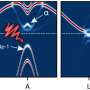
Glasses begin to reveal their high pressure secrets

The structural changes in glasses and liquids induced by high-pressure conditions can substantially alter their dynamical and transport properties. Unravelling the mechanisms behind these transformations is, however, a formidable task owing to the intrinsic disorder of the atomic arrangements and the need for small samples to attain high pressure conditions. In J. Phys.: Condens. Matter 24 502101, we show that neutron diffraction with isotope substitution can be used to disentangle the structural complexity of glass at pressures up to 8 GPa (80,000 atmospheres). The work reveals the nature of density-driven structural collapse. A new probe for the structure of materials under extreme conditions is introduced.
Neutron diffraction with isotope substitution has played a key role in revealing the structure of multi-component glasses and liquids. However, there are a number of challenges at high-pressure: the sample sizes are necessarily small; the high-pressure apparatus leads to detrimental background scattering; and neutron diffraction is a flux-limited probe by comparison with x-ray diffraction. Recently, much effort has been devoted to the instrumentation and methodology required to make accurate measurements of the neutron diffraction patterns for glasses and liquids at pressures within the 1–20 GPa regime. The progress made has facilitated a first exploitation of high-pressure neutron diffraction with isotope substitution. The method was applied to amorphous GeO2 on account of its role as a prototypical network-glass former and the availability of suitable germanium isotopes.
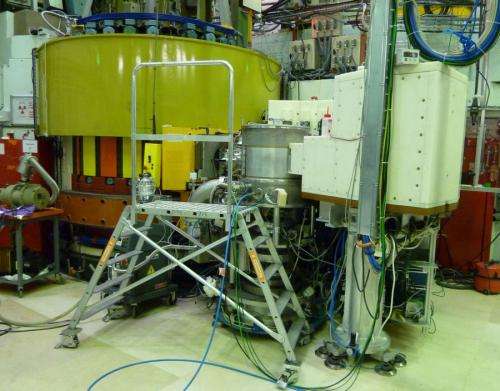
The experimental results, obtained at the Institut Laue-Langevin, enabled the coordination environments of Ge and O to be individually resolved over an extended range of distances in real space. In particular, they provided a rigorous test of various models proposed for the mechanisms of density-driven network collapse as the structural motifs transform from GeO4 tetrahedra to GeO6 octahedra. The experimental results are in quantitative agreement only with molecular dynamics simulations made using transferrable interaction potentials that include dipole-polarization effects. Unlike its crystalline counterpart, fivefold coordinated germanium atoms in GeO2 glass play an essential role in the pressure-driven transformations.
The work in J. Phys.: Condens. Matter 24 502101 shows that neutron diffraction with isotope substitution can be successfully applied to a prototypical glass at pressures above 1 GPa. The method enables the detailed structure around targeted chemical species to be measured over a large range of distances in real space. This provides essential and currently unavailable information for testing competing models for the density-driven collapse of materials. The work opens up a new vista of opportunity for unravelling the structural complexity of a wide range of glasses and liquids under high-pressure conditions.
More information: iopscience.iop.org/0953-8984/24/50/502101/
Provided by Institute of Physics













.jpg)