October 12, 2012 feature
Sponge-like graphene makes promising supercapacitor electrodes
(Phys.org)—While most of today's electric vehicles rely on batteries to store energy, supercapacitors have enjoyed significant improvements that have made them serious competitors to batteries. Batteries traditionally have the upper hand in terms of capacity, since supercapacitors' low capacities mean very short driving ranges for electric vehicles. Supercapacitors' biggest advantage lies in their much higher power density compared to batteries, enabling a quicker charge time and the ability to quickly discharge for fast acceleration.
A new study by a group of researchers at the University of Alberta and the National Research Council of Canada, both in Alberta, Canada, has shown that supercapacitors have great potential for continuing improvements.
The researchers have synthesized a new material that they call sponge-like graphene due to its 3D macroporous structure and demonstrated that it can be used to make supercapacitor electrodes. Supercapacitors with these new electrodes have fair energy density when operating at low power densities, but their biggest attraction is when operating at ultrahigh power densities of around 48,000 W/kg, where they're able to deliver an attractive energy density of 7.1 Wh/kg.
At first, an energy density of 7.1 Wh/kg may not sound remarkable compared to the energy density of the best Li-ion batteries, such as Envia System's record-breaking 400 Wh/kg announced earlier this year. However, in order to reduce the time it takes to charge Li-ion batteries for electric vehicles from hours to minutes, batteries need to have a higher power density than their current best values of around 10,000 W/kg. So the 48,000 W/kg power density of the supercapacitors reported here, coupled with a 7.1 Wh/kg energy density, shows that supercapacitors may offer batteries some competition.
"Supercapacitors and batteries are quite different electrochemical energy storage devices," coauthor Zhi Li, of both the University of Alberta and the National Research Council of Canada, told Phys.org. "Here is an example used quite often to demonstrate their differences. If you are driving an electric vehicle, you would like a high-energy-density battery to keep the vehicle running for many miles and you would probably also prefer a high-power-density supercapacitor to make the car start/accelerate faster. Supercapacitors are designed to work at much higher power density (fast charging/discharging). 7.1 Wh/kg is far from attractive for a battery. However, this energy is delivered in less than 2 seconds. I believe none of the existing batteries is ready to do that."
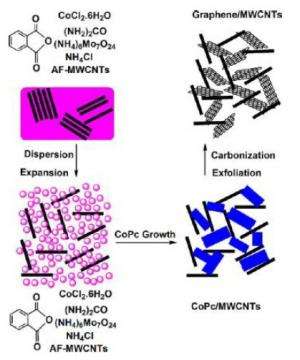
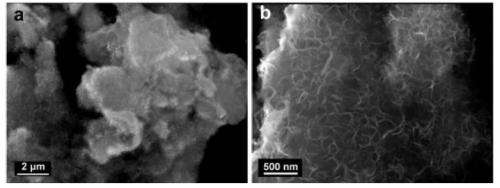
As the researchers explain in their study, they synthesized the sponge-like graphene out of multiwalled carbon nanotubes and cobalt phthalocyanine (PC) molecules that attach to nucleation sites in the nanotube "skeleton." These materials were microwave-heated for 20 minutes to yield graphite, and then immediately quenched with ice water to transform the graphite into graphene flakes. Scanning electron microscope images revealed a very uniform sponge-like morphology in the carbon structure.
In experiments, the researchers demonstrated that electrodes made of the sponge-like graphene are stable in two common electrolytes (ionic liquid and aqueous) used in supercapacitors. While many supercapacitor electrodes perform well only at temperatures of 60 °C (140 °F) or higher, the sponge-like graphene electrodes work very well at room temperature. The researchers attribute both the good room-temperature operation and the ability for fast electrolyte transfer (and resulting high power density) to the electrode's sponge-like macroporous structure.
The sponge-like graphene electrodes also exhibit an excellent cycle life. After running through 10,000 charge-discharge cycles, the electrodes retained 90% of their capacity in the ionic liquid electrolyte and 98% in the aqueous electrolyte.
"In this work, we grow graphene between CNTs and obtain a nano-architecture able to deliver energy at a super high power density," Li said. "However, the most significant contribution of the work is that we demonstrated a method suitable for making graphene in the limited space of other nanomaterials. PCs, the starting materials we used, are small molecules less than 2 nm and can fit in the tiny space of other nanomaterials. After carbonization and quenching, PCs are in situ converted into graphene. In addition, this conversion is a self-catalyzed reaction which offers great flexibility to make graphene compositing with other nanomaterials. As you know, the graphene composites have much wider application than graphene itself."
Overall, the results build on previous research demonstrating that 3D graphene structures may serve as an ideal structure for supercapacitor electrodes by allowing fast electrolyte transfer through the porous channels. The researchers hope that further improvements in the future will make supercapacitors attractive for electric vehicles, power back-up systems, and other high-power applications.
"We are seeking a way to make the graphene thinner, which would give the nano-architectures more energy density," Li said. "The current thickness of the graphene is about 5-6 layers. Our aim is to make it less than 2 layers. That will double or triple the energy density of the materials without sacrificing the power density."
More information: Zhanwei Xu, et al. "Electrochemical Supercapacitor Electrodes from Sponge-like Graphene Nanoarchitectures with Ultrahigh Power Density." The Journal of Physical Chemistry Letters 2012, 3, 2928-2933. DOI: 10.1021/jz301207g
Journal information: Journal of Physical Chemistry Letters
Copyright 2012 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.