Electrons doing the splits
Observations of a 'single' electron apparently splitting into two independent entities -- so-called quasi-particles -- are reported in this week’s Nature.
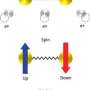
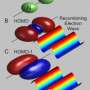
An electron has been observed to decay into two separate parts, each carrying a particular property of the electron: a spinon carrying its spin - the property making the electron behave as a tiny compass needle - and an orbiton carrying its orbital moment - which arises from the electron's motion around the nucleus. These newly created particles, however, cannot leave the material in which they have been produced. This result is reported in a paper published in Nature by an international team of researchers led by experimental physicists from the Paul Scherrer Institute (Switzerland) and theoretical physicists from the IFW Dresden (Germany).
All electrons have a property called "spin", which can be viewed as the presence of tiny magnets at the atomic scale and which thereby gives rise to the magnetism of materials. In addition to this, electrons orbit around the atomic nuclei along certain paths, the so-called electronic "orbitals". Usually, both of these quantum physical properties (spin and orbital) are attached to each particular electron. In an experiment performed at the Paul Scherrer Institute, these properties have now been separated.
The electron's break-up into two new particles has been gleaned from measurements on the copper-oxide compound Sr2CuO3. This material has the distinguishing feature that the particles in it are constrained to move only in one direction, either forwards or backwards. Using X-rays, scientists have lifted some of the electrons belonging to the copper atoms in Sr2CuO3 to orbitals of higher energy, corresponding to motion of the electron around the nucleus with higher velocity. After this stimulation with X-rays, the electrons split into two parts. One of the new particles created, the spinon, carries the electron's spin and the other, the orbiton, the increased orbital energy. In this study, the fundamental spin and orbital moments have been observed, for the first time, to separate from each other.
In the experiment, X-rays from the Swiss Light Source (SLS) are fired at Sr2CuO3. By comparing the properties (energy and momentum) of the X-rays before and after the collision with the material, the properties of the newly produced particles can be traced. "These experiments not only require very intense X-rays, with an extremely well-defined energy, to have an effect on the electrons of the copper atoms", says Thorsten Schmitt, head of the experimental team, "but also extremely high-precision X-ray detectors. In this respect, the SLS at the Paul Scherrer Institute is leading the world at the moment."
"It had been known for some time that, in particular materials, an electron can in principle be split", says Jeroen van den Brink, who leads the theory team at the IFW Dresden, "but until now the empirical evidence for this separation into independent spinons and orbitons was lacking. Now that we know where exactly to look for them, we are bound to find these new particles in many more materials."
Observation of the electron splitting apart may also have important implications for another current research field - that of high-temperature superconductivity. Due to the similarities in the behaviour of electrons in Sr2CuO3 and in copper-based superconductors, understanding the way electrons decay into other types of particles in these systems might offer new pathways towards improving our theoretical understanding of high-temperature superconductivity.
More information: Spin-Orbital Separation in the quasi 1D Mott-insulator Sr2CuO3, J. Schlappa, K. Wohlfeld, K. J. Zhou, M. Mourigal, M. W. Haverkort, V. N. Strocov, L. Hozoi, C. Monney, S. Nishimoto, S. Singh, A. Revcolevschi, J.-S. Caux, L. Patthey, H. M. Rønnow, J. van den Brink, and T. Schmitt; Nature, Advance Online Publication, 18.04.2012, DOI: 10.1038/nature10974
Journal information: Nature
Provided by Paul Scherrer Institute