Nanotechnology holds promise for safer breast implants
A new review published in WIREs Nanomedicine and Nanobiotechnology explores how nanotechnology may be used to develop safer breast implants as an alternative to silicone rubber, minimizing health complications.
Around 75% of post-mastectomy patients elect some form of breast reconstruction. The only material option available to women undergoing breast reconstruction and augmentation is based on silicone rubber. While no medical device is 100% safe and effective, there is an extraordinarily high rate of complications reportedly attributed to silicone breast implants (20-30% - no other medical device has such a high failure rate), including increased incidence of systemic diseases, various forms of cancer, and psychological disease.
Lead review Author Judit E. Puskas, Ph.D., M.E., of The University of Akron, and researchers surveyed the literature on breast implants from the perspective of material science to determine how nanotechnology may enable the future development of safer breast implants.
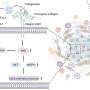
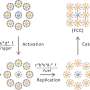
By reducing the size of the components in nanostructured materials, unprecedented properties can be achieved. The Authors are currently developing an alternative nanostructured material to silicone rubber that will minimize complications.
The new material will also be able to deliver cancer drugs locally to improve the efficacy of treatment and minimize side effects associated with chemotherapy.
"If successful, our material could be used for implants with drug delivery capabilities," Puskas notes. "We are hoping that this review will contribute to a better understanding of the controversial issues and motivate material scientists and medical doctors to work together to develop alternatives based on new nanotechnology for the women who opt for a device made of synthetic materials."
Provided by Wiley