Abrupt escape from flatness
At first glance, it seems as if billions of lead atoms have mysteriously disappeared. When exposed to heat, a layer of lead coated onto a nickel surface becomes almost invisible from one moment to the next. In reality, the slightest disturbance causes these atoms to suddenly switch from a broad “flat pancake” shape to a compact hemisphere. This remarkable phenomenon was first revealed by researchers at the University of Twente’s MESA+ Institute for Nanotechnology, who have since published their results in Physical Review Letters.
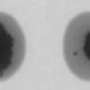
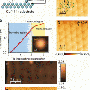
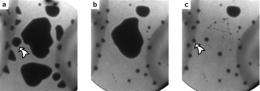
A lead coating on a nickel surface has unusual electronic properties which cause it to form flat "pancakes", consisting of billions of atoms arranged in a crystalline structure. These "pancakes" of solid lead are quantum mechanically stabilized and just a couple of dozen atoms thick. When exposed to gradual heating, nothing much changes at first. At about 520 Kelvin (247 degrees Celsius), however, the lead coating suddenly seems to disappear completely. Within the space of a few milliseconds, the lead "slivers" transform into hemispheres with a radius (or "height") of a few micrometers. Interestingly, this all takes place at a temperature below the melting point of lead. The hemispheres, too, consist of solid lead. So no mass has been lost, the material has simply taken on a different spatial configuration.
The technique used by the researchers to observe this process is known as Low Energy Electron Microscopy (LEEM). There are only a few such microscopes in existence, but two have recently been installed in the Netherlands. They are designed to bombard surfaces with low energy electrons. This makes them especially well suited to making accurate observations of surface phenomena and events in thin films.
The abrupt transformation from flat to spherical can be explained in terms of the most energetically favourable shape. From this viewpoint, hemispheres make much more effective use of surfaces, whereas pancakes are not very stable. There has recently been a massive expansion in our understanding of atomic processes right down to the level of single atoms, facilitated by experimental techniques such as Scanning Tunnelling Microscopy (STM), together with newly developed theories. Even so, we cannot account for the sheer speed at which this transition takes place.

However, this recently discovered super-fast transition from two to three dimensions is based on a delicate interplay between several atoms, a kind of group process. In their published article, these researchers from Twente express the view that a more detailed explanation of the very rapid transition from flat to spherical will only be possible when we have a better fundamental theoretical understanding of meso-level phenomena. LEEM can be used to make direct observations of new phenomena at the meso-scale, thereby generating data crucial to our knowledge of this field. The importance of these results is that they will give us a more profound understanding of the stability of nanostructures.
The article entitled "Anomalous decay of electronically stabilized lead mesas on Ni(111)" by Tjeerd Bollmann, Raoul van Gastel, Harold Zandvliet and Bene Poelsema has been published in Physical Review Letters. This September, Tjeerd Bollmann successfully defended his PhD thesis entitled "Escape from Flatland", which was supervised by Prof. Bene Poelsema (UT) and Prof. Joost Frenken (UL).
Provided by University of Twente