Exposing ZnO nanorods to visible light removes microbes
The practical use of visible light and zinc oxide nanorods for destroying bacterial water contamination has been successfully demonstrated by researchers at the Asian Institute of Technology (AIT). Nanorods grown on glass substrates and activated by solar energy have been found to be effective in killing both gram positive and gram negative bacteria – a finding that has immense possibilities for affordable and environmentally friendly water purification techniques.
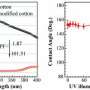
"Most studies so far either work on the use of ultraviolet light or involve a suspension of nanoparticles," revealed Prof. Joydeep Dutta, director of the Center for Excellence in Nanotechnology at AIT. The AIT research group has dispensed with both. Instead of using a suspension of nanoparticles, which have to be removed later after the water purification process, or relying on UV light, the group demonstrated a system featuring visible light and ZnO nanorods. "The key concept was to incorporate deliberate defects in ZnO nanorods by creating oxygen vacancies and interstitials, which then allows visible light absorption," he explained.
Environmentally friendly approach
Such ZnO nanorods grown on glass were tested on Escherichia coli and Bacillus subtilis bacteria, which are commonly used as model microbes. In the dark, ZnO dissolves slowly releasing zinc ions, which have anti bacterial properties, as it penetrates the bacterial cell envelope thereby thwarting the growth of microbes. Under well lit conditions, the effect is doubled with both photocatalysis and zinc ions playing their part in killing the microbes.
The implications of these experiments are enormous. "Since ZnO has now been tested under solar light, instead of the traditionally used UV light, the potential for commercial applications is huge, particularly since the levels of zinc ions removed from the rods to the water are safe for human consumption," added Dutta.
The team, which also includes Dr. Oleg V Shipin, Ajaya Sapkota, Dr. Alfredo J Anceno, Mr. Sunandan Baruah and Ms. Mayuree Jaisai, is continuing its work on photocatalysis for use in water decontamination.
More information: The original research can be read in the journal Nanotechnology at this link iopscience.iop.org/0957-4484/22/21/215703/
Provided by Asian Institute of Technology