Predicting world's smoggiest days with NASA technology

A research team led by NASA's Jet Propulsion Laboratory and the California Institute of Technology (Caltech), both in Pasadena, Calif., has fully characterized a key chemical reaction that affects the formation of pollutants in smoggy air in the world's urban areas. When applied to Los Angeles, the laboratory results suggest that, on the most polluted days and in the most polluted parts of L.A., current models are underestimating ozone levels by 5 to 10 percent.
The results-published this week in the journal Science-are likely to have "a small but significant impact on the predictions of computer models used to assess air quality, regulate emissions and estimate the health impact of air pollution," said Mitchio Okumura, professor of chemical physics at Caltech and one of the principal investigators on the research.
"This work demonstrates how important accurate laboratory measurements are to our understanding of the atmosphere," said JPL senior research scientist Stanley P. Sander, who led the JPL team's effort. "This is the first time this crucial chemical reaction has been studied by two teams using complementary methods that allow its details to be understood."
The key reaction in question in this research is between nitrogen dioxide and the hydroxyl radical. In the presence of sunlight, these two compounds, along with volatile organic compounds, play important roles in the chemical reactions that form ozone, which at ground-level is an air pollutant harmful to plants and animals, including humans.
Until about the last decade, scientists thought these two compounds only combined to form nitric acid, a fairly stable molecule with a long atmospheric life that slows ozone formation. Chemists suspected a second reaction might also occur, creating peroxynitrous acid, a less stable compound that falls apart quickly once created, releasing the hydroxyl radical and nitrogen dioxide to resume ozone creation. But until now they weren't sure how quickly these reactions occur and how much nitric acid they create relative to peroxynitrous acid. The JPL team measured this rate using a high-accuracy, JPL-built, advanced chemical reactor. The Caltech team then determined the ratio of the rates of the two separate processes.
Theoretical calculations by chemistry professor Anne McCoy at Ohio State University, Columbus, contributed to understanding of the not-well-studied peroxynitrous acid molecule.
"This work was the synthesis of two very different and difficult experiments," added lead author and former Caltech graduate student Andrew Mollner with Aerospace Corporation, El Segundo, Calif. "While neither experiment in isolation provided definitive results, by combining the two data sets, the parameters needed for air quality models could be precisely determined."
In the end, the researchers found the loss of hydroxyl radical and nitrogen dioxide is slower than previously thought-although the reactions are fast, fewer of the radicals are ending up as nitric acid than had been supposed, and more of them are ending up as peroxynitrous acid. "This means less of the hydroxyl radical and nitrogen dioxide go away, leading to proportionately more ozone, mostly in polluted areas," Okumura said.
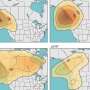
Just how much more? To try to get a handle on how their results might affect predictions of ozone levels, they turned to Robert Harley, professor of environmental engineering at the University of California, Berkeley, and William Carter, a research chemist at the University of California, Riverside-both experts in atmospheric modeling-to look at the ratio's impact on predictions of ozone concentrations in various parts of Los Angeles in the summer of 2010.
The result: "In the most polluted areas of L.A.," said Okumura, "they calculated up to 10 percent more ozone production when they used the new rate for nitric acid formation."
Okumura said this strong effect would only occur during the most polluted times of the year, not all year long. Still, he said, considering the significant health hazards ozone can have-recent research has reported that a 10 part-per-billion increase in ozone concentration may lead to a four percent increase in deaths from respiratory causes-any increase in expected ozone levels will be important to people who regulate emissions and evaluate health risks. The precision of these results reduces the uncertainty in the models-an important step in the ongoing effort to improve the accuracy of models used by policymakers.
Okumura believes this work will cause other scientists to reevaluate recommendations made to modelers on the best parameters to use. For the team, however, the next step is to start looking at a wider range of atmospheric conditions where this reaction may also be important.
Sander agrees. "The present work focused on atmospheric conditions related to urban smog-i.e., relatively warm temperatures and high atmospheric pressure," he said. "But the hydroxyl radical/nitrogen dioxide reaction is important at many other altitudes. Future work by the two groups will focus on the parts of the atmosphere affected by long-range transport of pollution by high-altitude winds [in Earth's middle and upper troposphere] and where ozone depletion from human-produced substances is important [the stratosphere]."
Provided by JPL/NASA