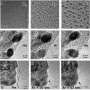
The effects of hydrogen on growing carbon nanotubes
Carbon nanotubes -- long, hollow cylinders of carbon billionths of a meter in diameter -- have many potential uses in nanotechnology, optics, electronics, and many other fields. The exact properties of nanotubes depend on their structure, and scientists as yet have little control over that structure, which is determined during the initial formation -- or growth -- of the nanotubes. In fact, says chemical engineer and materials scientist Eray Aydil of the University of Minnesota, "we do not know precisely how the nanotubes grow."
In a paper in the American Institute of Physics' Journal of Applied Physics, Aydil, professor of chemical engineering and materials science and the Ronald L. and Janet A. Christenson Chair in Renewable Energy, and his colleagues shed new light on the process. In particular, the researchers examined the influence of hydrogen gas.
"Carbon nanotubes grow from a metal catalyst particle that is immersed in a gas like methane," Aydil explains. "Sometimes hydrogen gas is also added and it was found that a little bit of hydrogen helps to grow carbon nanotubes with nice straight walls and with few defects. However, too much hydrogen addition gives fibers with thick walls, instead of nanotubes, or no growth at all."
To understand why, Aydil and colleagues used transmission electron microscopy and other methods to systematically image and characterize the effects of increasing concentrations of hydrogen. "It turns out that the iron metal catalysts turn to iron carbide by reacting with the carbon in methane. Iron carbide is a hard material that is not easily deformed, and carbon nanotubes grown from such catalysts tend to have nice straight walls," he says.
Adding more hydrogen to the mix causes iron carbide to turn into iron -- which is more malleable and ductile, and "deforms into shapes that give rise to more fiber-like structures rather than hollow carbon nanotubes," he says. At higher concentrations, hydrogen etches the forming carbon nanotubes, "and growth stops all together. It is the interaction of the hydrogen with the catalysts and its effect on the catalyst's structure that controls the carbon nanotube structure."
More information: The article, "Effect of Hydrogen on Catalyst Nanoparticles in Carbon Nanotube Growth" by Eray S. Aydil, Michael J. Behr, Elizabeth A. Gaulding and K. Andre Mkhoyan (University of Minnesota) appears in the Journal of Applied Physics. link.aip.org/link/japiau/v108/i5/p053303/s1
Provided by American Institute of Physics