Artificial metalloenzymes, the chemical synthesis of the future
Researchers at CEA, Joseph Fourier University and CNRS have developed a new approach combining protein crystallography and biomimetic chemistry for observing they key steps of a process essential to life: oxygen activation. This was achieved by creating a complex artificial metalloenzyme composed of a chemical catalyst and a protein and observing it via X-ray crystallography at the European Synchrotron Radiation Facility (ESRF). The results obtained constitute an essential step towards the development of artificial metalloenzymes capable of producing various molecules of industrial interest more efficiently and at a lesser cost, thereby opening new prospects for green chemistry. These results are published online by Nature Chemistry.
A large number of chemical molecules exist in two forms with inverted, mirror-like structures (enantiometers). In many cases, only one of the two molecular forms is of interest for the healthcare, agricultural or food industries. Molecular chemical synthesis has the disadvantage of producing both molecular forms (enantioselective catalysis), and therefore a non-negligible quantity of molecules without interest. In order to isolate the form of interest, complex and expensive purification phases need to be implemented.
Nature is far more efficient than chemical synthesis. Indeed, enzymes are generally capable of directly producing the molecular form of interest. Hence the idea of using them for industrial applications. However, the number of natural enzymes available to produce the reactions of interest remains low. Homogeneous chemical catalysts can be used to produce a large number of reactions, but often with low catalyst stability and lack of specificity.
This has led to the idea of combining chemistry and biology to create artificial metalloenzymes. Their structure consists of an inorganic catalyst incorporated in an inactive protein structure. Each constituent plays its part: the inorganic catalyst determines the nature of the reaction by acting as the active site, and the protein structure controls the production of the molecular form of interest and the efficiency of the reaction.
Although, conceptually speaking, these artificial metalloenzymes offer huge prospects for green chemistry, there still remains the technological challenge of engineering efficient enzymes for producing each molecule of interest. This entails identifying the best protein/catalyst pair, understanding its functioning, adapting it, etc.
By developing a method to observe the progress of the chemical reaction in the active site over time, these researchers have completed an essential step in the development of metalloenzymes. "At present, we have observed the functioning of a molecular oxygen activation reaction. This reaction is used in a large number of cellular processes essential to life", explains Stéphane Ménage, CNRS researcher of the Bioinspired Redox Chemistry team at the Life Sciences and Technologies Research Institute (IRTSV).
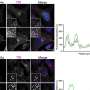
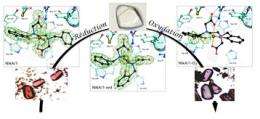
In order to study this reaction, the researchers have mimicked it by introducing an aromatic cycle in an iron complex and then incorporating this complex into a protein whose sole function is nickel transport in Escherichia coli bacteria. This protein therefore does not disturb the chemical oxygen activation reaction. The researchers have then crystallized this artificial metalloenzyme and directly observed the evolution of the reaction within the crystal via X-ray crystallography. "The crystal enables the diffusion of the reaction substrates and intermediaries. The enzyme remains active in the crystal, and the various steps of the reaction can be observed directly therein", explains Christine Cavazza, CEA researcher at the Protein Crystallography and Crystallogenesis Laboratory of the Institute of Structural Biology (IBS). "We can thus observe the incorporation of oxygen atoms into an aromatic core".
"The most extraordinary aspect for us chemists is that this combination of chemical and biological properties has enabled us to observe all the steps of this reaction, something no chemist had previously achieved", explains Stéphane Ménage. "This is essential for studying the functioning of the chemically synthesized active site. We can then adapt its structure according to the characteristics sought".
More information: Crystallographic snapshots of the reaction of aromatic C–H with O2 catalysed by a protein-bound iron complex. Christine Cavazza, et al. Nature Chemistry, online, 2010.
Provided by CNRS