Self-repairing materials
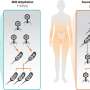
Will the day come when cracks in buildings close up without external help and before they get to the stage where they cause damage to the component? This might appear utopia, but it already occurs in nature. When a person suffers a minor wound, the human body reacts to close the opening, sending the blood platelets needed to the affected area – and with no need in many cases for any external coagulant substance to be employed.
This reaction of nature to damage suffered was the starting point for the development of self-repairing polymer materials with the capacity of recovering a good part of the properties lost and with no or with minimal external help. In the case of ceramics or metallic materials, progress is much slower, being limited to initial steps.
There are currently two notable self-repairing technologies in polymer materials: adhesives and thermal encapsulation.
As the name suggests, the first of these involves a series of "stores" of adhesive found distributed in the most homogenous manner possible throughout the material, so that when the crack reaches one of these nodes the adhesive is secreted, together with a catalyst, and the crack is closed and the material polymerised.
There are two variants within this line of technology, depending on whether adhesive-containing microcapsules or tubes filled with adhesive are employed.
INASMET-Tecnalia has worked on this line in a project undertaken for the AIRBUS, having managed to produce a series of microcapsules and distribute them in a polymeric resin. This was a fundamental step to finding out the difficulties that might arise in the encapsulation process.
The second method, developed by Bristol University, is a project for the ESA, is very similar. The difference lies in the use of tubes rather than microcapsules filled with adhesive.
The thermal method uses a different repair methodology. The material, developed by the University of Sheffield, is a polymeric matrix compound, reinforced with carbon fibres. The polymer matriz, in turn, is made of a solid solution of a thermoplastic polymer and another thermostable polymer.
The only restriction of the thermostable material is that it has to be suitable for incorporating these reinforcment fibres into it. The thermoplastic material has greater limitations, limiting it chances of being chosen for use, being highly dependant on the thermostable material used. In this case, when damage is detected, repair is carried out by heating the material with some device incorporated into it.
This heating is capable of raising the temperature above that of the fusion of the thermoplastic material which, as a result, melts and flows into the damaged areas so that the cracks are sealed and the component restored to its former condition. INASMET-Tecnalia has also worked in this field within the framework of the aforementioned project.
It should be underlined that the development of self-repairing materials is still at initial stages and there is a long way to go yet before reaching the desired goal. Nevertheless, the results obtained are encouraging.
Apart from participation in this project, INASMET-Tecnalia is working on a number of research lines related to the growing demand that is anticipated for self-repairing materials.
Source: Elhuyar Fundazioa