Detecting Disease
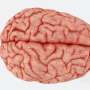
![True color fluorescence microscopy images of dual-function beads embedded with iron oxide and (A) 630 nm emission QDs and (B) 580 nm emission QDs. The beads are coated with an amphiphilic polymer and soluble in water. Images were taken at 60x magnification under blue light excitation. [Sathe and Nie, Development of dual-function microbeads embedded with quantum dots and iron oxide nanocrystals for biomedical applications, SPIE Photonics West, 2007, 6448-6.] Detecting Disease](https://scx1.b-cdn.net/csz/news/800a/2007/detectingdis.jpg)
Analyzing human blood for a very low virus concentration or a sample of water for a bioterrorism agent has always been a time-consuming and difficult process. Researchers at the Georgia Institute of Technology and Emory University have developed an easier and faster method to detect these types of target molecules in liquid samples using highly porous, micron-sized, silica beads.
The researchers developed a technique to simultaneously or sequentially add optical and magnetic nanoparticles into the beads. Adding magnetic nanoparticles allows the use of a magnetic field to attract and easily remove the beads from a liquid sample.
“These nanoparticles enter the pores of the microbeads so quickly and so completely -- essentially more than 99 percent of the nanoparticles go into the pores of the beads,” explained Shuming Nie, the head researcher on the project and the Wallace H. Coulter Distinguished Chair in Biomedical Engineering and director of Emory-Georgia Tech Nanotechnology Center.
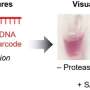
The beads are mixed in a liquid such as urine. Viruses, proteins or other biomarkers are captured on the bead surface. After the beads are removed from the liquid, optical imaging is used to determine the concentration of a specific protein or virus in the liquid sample based on the number of proteins or viruses attached to the surface of the beads.
Tushar Sathe, a graduate student in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University, described the process of creating these novel beads and their clinical applications on Jan. 20 at SPIE Photonics West in San Jose, California. The work was also published in the Aug. 15 issue of Analytical Chemistry.
The technology involves embedding fluorescent quantum dots and magnetic iron oxide nanoparticles inside the beads to create dual-modality magneto-optical beads. Nie and Sathe synthesize the quantum dots in different colors by varying their size, giving the beads a unique optical signature. Having different color beads allows the researchers to detect several target molecules at the same time in the same liquid sample.
“We use the quantum dots to create a set of beads that are unique and can be distinguished from each other. It’s similar to bar-coding -- once you barcode the beads and put them in the urine or blood sample, you can remove them and decode what proteins or viruses have attached to individual beads based on their spectral signature,” explained Sathe.
The process of creating these beads is quite simple, according to Sathe. The surface of the beads contains a long-chain carbon molecule that makes the beads hydrophobic, meaning they repel water. The beads are dissolved in butanol and washed several times. Then the beads are counted and optical and magnetic nanocrystals are added to the suspension either simultaneously or sequentially.
After 15-20 minutes, the butanol is removed to get rid of any remaining nanoparticles that didn’t get incorporated into the beads and the beads are washed with ethanol. Then the beads are coated with a polymer that creates a hydrophilic surface on the beads. This allows the beads to be functionalized by adding antibodies or DNA molecules to the surface that will capture the target molecules.
These beads are dual-function -- both optical and magnetic -- but according to Sathe, more functions can be added to the beads. “Adding them is as easy as adding the nanoparticles into the solution. You just have to make sure the nanoparticle surface is hydrophobic so that it interacts with the beads,” said Sathe.
The primary biomedical applications for this new technology will be to detect cancer and neurological diseases by identifying certain molecules present in human blood or urine that indicate specific diseases, according to Nie, who is also professor of biomedical engineering, chemistry, materials science & engineering, and hematology and oncology at Emory University and the Georgia Institute of Technology.
“Some of the biomarkers for Alzheimer’s disease have very low concentrations in the blood so you need highly sensitive techniques that can find a specific molecule to diagnose this disease,” explained Nie. “Our technique could also be used to monitor therapeutic response. For example, if the viral level decreases in samples taken at later dates, then we know the drug is probably working.”
This new technology allows the researchers to analyze very low concentrations of target molecules. “Instead of analyzing a liter of sample where the concentration could be very dilute and you might not see the target molecule you’re looking for, you can let the beads capture the molecules on their surface, remove them from the liquid, and then just measure the number of molecules attached to the beads,” said Nie.
Source: Georgia Institute of Technology, by Abby Vogel