Researchers develop 'MRI' for fuel cells
As gasoline prices top $3 a gallon in major cities, the drive toward increasing energy efficiency and reducing air pollution has accelerated, and the development of fuel cells has become a major focus worldwide.
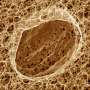
Knowing how fuel cells work is key to improving their performance and reducing the cost of their production. Now a research team led by Scott A. Barnett, professor of materials science and engineering at Northwestern University, has produced the first three-dimensional images of the interior of a fuel cell -- providing a new tool for the study and development of fuel cells.
The researchers' three-dimensional reconstruction of a solid oxide fuel cell anode was reported in a paper published this month by the journal Nature Materials. (A solid oxide fuel cell efficiently converts fuels such as hydrogen and natural gas directly into electricity; Barnett's group also recently reported a similar fuel cell that works with a liquid transportation fuel -- iso-octane, a high-purity compound similar to gasoline.)
"Much like magnetic resonance imaging produces a view inside the human body, we now can look inside fuel cells," said Barnett. "The dual-beam focused-ion-beam microscope used in the study provides much higher resolution than an MRI, showing nanometer-scale features. These pictures will help us and other researchers to unravel how fuel cells work so they can eventually be improved and made to work longer without failing."
The imaging technique also will enable manufacturers to maintain quality by checking batches of fuel cells for any structural changes that might hurt the fuel cells' characteristics.
The materials comprising fuel cells have become increasingly sophisticated, both in composition and microstructure. Determining this microstructure is a critical, yet usually missing, link between materials properties and processing and electrode performance, said Barnett. Current methods of microstructural analysis, such as scanning electron microscopy, provide only two-dimensional images of the microstructure, limiting understanding of how regions are interconnected in three-dimensional space.
A fuel cell is like a battery that can be replenished with fresh fuel. It consists of two electrodes sandwiched around an electrolyte material that conducts ions between them. Oxygen enters at the cathode, where it combines with electrons and is split into ions that travel through the electrolyte to react with fuel at the anode. Fuel cells are environmentally friendly: water and carbon dioxide are the only by-products. In the process, the oxygen ions traversing the electrolyte produce a useful current.
Source: Northwestern University