When gold becomes a catalyst
Gold has always been perceived as a precious material: you win a gold medal when you prove to be the best in a competition; you only get a Gold credit card when you are a preferential customer, and the jewelry made of this material is amongst the most valuable. However, gold has also unexpected properties: It can act as a catalyst and transform carbon monoxide (CO) to carbon dioxide (CO2) when it comes in the form of tiny pieces, called nano-particles.
Gold suddenly enhances desired chemical reactions as a catalyst for example in the removal of odours and toxins or to clean automotive exhaust gases. Researchers from Switzerland, UK, the USA and the ESRF (Grenoble) have monitored the catalytic process and proposed an explanation for the high catalytic activity of gold. They publish today their results in the journal Angewandte Chemie online.
The team used nano-particles of gold instead of bulk gold. The catalyst structure looks as if someone had pulverized a piece of gold and spread the tiny nano-sized pieces over an aluminum oxide support. The properties of the nano-particles are very different from those of bulk gold. Only when the gold atoms are confined to the size of just a few millionth of a millimetre they start showing the desired catalytic behaviour.
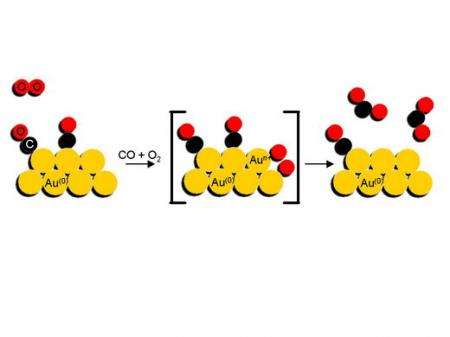
Scientists already knew that gold nano-particles react with this kind of setup and catalyses CO with oxygen (O2) into CO2. What they did not know was how the oxygen is activated on the catalyst. In order to find that out, they set up a cell where they could carry out the reaction, and in situ perform an X-ray experiment with the ESRF beam.
The researchers first applied a flow of oxygen over the gold nano-particles and observed how the oxygen becomes chemically active when bound on the gold nano-particles using high-energy resolution X-ray absorption spectroscopy. While constantly monitoring the samples, they switched to a flow of toxic carbon monoxide and found that the oxygen bound to the gold reacted with the carbon monoxide to form carbon dioxide. Without the gold nano-particles, this reaction does not take place. “We knew beforehand that the small gold particles were active, but not how they did the reaction. The nice thing is that we have been able to observe, for the first time, the steps and path of the reaction. The results followed almost perfectly our original hypotheses. Isn’t it beautiful that the most inert bulk metal is so reactive when finely dispersed?” comments Jeroen A. van Bokhoven, the corresponding author of the paper.
The possible applications of this research could involve pollution control such as air cleaning, or purification of hydrogen streams used for fuel cells. “Regarding the technique we used, the exceptionally high structural detail that can be obtained with it could be used to study other catalytic systems, with the aim of making them more stable and perform better”, says van Bokhoven.
One of the great advantages of this experiment is the nature of catalysis. The fact that once the material has reacted, it goes back to its initial state, has made the experiments easier. Nevertheless, in technological terms, it has been very demanding: “We combined the unique properties of our beamline with an interesting and strongly debated question in catalysis. Some extra time was needed to adapt the beamline, to the special requirements of this experiment,” explains Pieter Glatzel, scientist in charge of ID26 beamline, where the experiments were carried out. At the end, it only took the team a bit over half a year to prepare and carry out the experiments and publish the paper. “This is a very nice recognition of our work,” says Glatzel.
The article appears in this week’s international edition of Angewandte Chemie with a very high impact among the chemistry audience. In addition to this, the paper has been attributed the status of Very Important Paper, which is given to only 5% of all the publications in this journal.
Source: European Synchrotron Radiation Facility