New laser technique that strips hydrogen from silicon surfaces
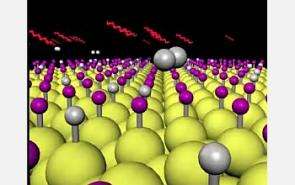
A team of researchers have achieved a long-sought scientific goal: using laser light to break specific molecular bonds. The process uses laser light, instead of heat, to strip hydrogen atoms from silicon surfaces, a key step in the manufacture of computer chips and solar cells.
The new technique was developed by Philip Cohen, a professor of electrical and computer engineering at the University of Minnesota, working with Vanderbilt University researchers Leonard Feldman, Norman Tolk and Zhiheng Liu along with Zhenyu Zhang from Oak Ridge National Laboratory. It is described in the May 19 issue of the journal Science.
"We live in the silicon age," said Tolk, who is a physics professor at Vanderbilt. "The fact that we have figured out how to remove hydrogen with a laser raises the possibility that we will be able to grow silicon devices at very low temperatures, close to room temperature."
Microelectronic devices are built from multiple layers of silicon. In order to keep silicon surfaces from oxidizing, semiconductor manufacturers routinely "passivate" them by exposing them to hydrogen atoms that attach to all the available silicon bonds. However, this means that the hydrogen atoms must be removed before new layers of silicon can be added. "Desorbing" the hydrogen is usually done by heating to high temperatures (800 C), which can create thermal defects in the chips and so reduce chip yields.
"One application that we intend to examine is the use of this technique to manufacture field effect transistors (FETs) that operate at speeds about 40 percent faster than ordinary transistors," said Cohen. According to Cohen, it should be possible to reduce the processing temperature of manufacturing FETs by 100 degrees Celsius, which should dramatically improve yields.
The research was carried out at Vanderbilt's W.M. Keck Free-electron Laser Center. The free-electron laser is a special kind of laser with the advantage that its beam can be tuned through a wide range of frequencies in much the same way that you can dial up different frequencies on a radio.
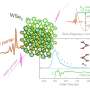
Because the silicon/hydrogen system has been intensively studied, the researchers knew the strength of the bond between the silicon and hydrogen atoms. The bonds between atoms act something like an atomic spring. Like tiny springs, they tend to vibrate at certain frequencies and are most likely to absorb light photons that vibrate at these frequencies. As a result, light tuned to these "resonant" frequencies can force the bond to break.
When the researchers scanned the laser through the frequencies that they had calculated would resonate with the silicon-hydrogen bond, they found that the rate of hydrogen desorption peaked at an incident wavelength of 4.8 microns (1/6,250th of an inch). They also tested the system on silicon surfaces covered with a mixture of hydrogen and deuterium. Deuterium is an isotope of hydrogen: Instead of the single proton that hydrogen has as a nucleus, deuterium has a proton and a neutron. It has the same chemical characteristics as hydrogen but it weighs about twice as much. This weight difference means that the silicon-deuterium bond vibrates more slowly than the silicon-hydrogen bond, so the resonant wavelength is very different than for hydrogen-silicon.
Prior theoretical work in collaboration with Baio Wu, then a postdoctoral fellow at Oak Ridge National Laboratory, predicted that a substantial fraction of the hydrogen could be excited but that temperatures well above room temperature would be needed for an effective process. But once they got the setup right, the researchers found that the laser desorption process:
-- Strips hydrogen from the silicon surface even at room temperature.
-- Generates surprisingly little heat. In the infrared wavelengths used by the researchers, silicon is basically transparent.
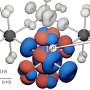
-- Exhibits a high degree of selectivity. With the hydrogen/deuterium mixture, the researchers demonstrated that they can remove large numbers of hydrogen atoms without detaching many of the deuterium atoms.
Selectivity of this kind could provide a way to control the growth of nanoscale structures with an unprecedented degree of precision, and it is this potential that most excites Cohen. "By selectively removing the hydrogen atoms from the ends of nanowires, we should be able to control and direct their growth, which currently is a random process," he said.
So far, three patent disclosures have been filed by the University of Minnesota, along with Vanderbilt University, on this process. At this point, the researchers can only speculate on the reasons why their technique succeeds where so many others have failed. The main clue is the totally unexpected observation that the hydrogen atoms appear to detach from the surface in pairs, as hydrogen molecules, rather than as individual atoms. Additional research will be needed to work out the atomic mechanism involved.
Source: University of Minnesota