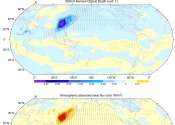
Atmospheric 'teleconnections' sustain warm blobs in the northeast Pacific Ocean
The past 10 years have seen a series of "warm blobs" in the northeast Pacific Ocean. These marine heat waves do widespread damage to ecosystems and marine life in the area, but the mechanisms by which they develop and are ...