The Indian villagers who lost their homes to the sea
The gentle roar of the ocean lulled Indian mother-of-two Banita Behra to sleep each night, until one day the encroaching tide reached her doorstep.

The gentle roar of the ocean lulled Indian mother-of-two Banita Behra to sleep each night, until one day the encroaching tide reached her doorstep.
Environment
5 hours ago
0
25

Water erosion is the most active process controlling soil formation and evolution, which can affect the redistribution of carbon between terrestrial, aquatic, and atmospheric ecosystems. Erosion-induced organic carbon dynamic ...
Earth Sciences
16 hours ago
0
1

New University of Minnesota research suggests "leaf glow" provides vital information on vegetation dynamics in Arctic and boreal ecosystems like Minnesota's forests and wetlands, which are among the fastest warming in the ...
Plants & Animals
16 hours ago
0
13
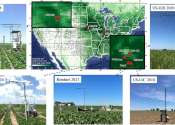
In recent years, the scientific community has increasingly turned its attention to sustainable agriculture, aiming to maximize crop yield while minimizing environmental impact. A crucial aspect of this research involves understanding ...
Ecology
17 hours ago
0
62

Arctic riverbanks are typically resilient, thanks to the power of permafrost. This permanently frozen soil locks in sediment, leading to low erosion rates. But as Arctic river water warms due to climate change, some researchers ...
Earth Sciences
19 hours ago
0
12

The Scottish government's decision to row back on its 2030 climate pledge illustrates the crux of any target: it's easy to set one with a big political flourish, but harder to follow through with a careful plan to achieve ...
Environment
20 hours ago
0
17

Researchers at the University of Bayreuth have gained new insights in the field of high-pressure carbon chemistry: They synthesized two new carbides—compounds of carbon and another chemical element—with unique structures. ...
Analytical Chemistry
21 hours ago
0
13

The Intergovernmental Panel on Climate Change (IPCC) has declared that removing carbon from the atmosphere is now essential to fighting climate change and limiting global temperature rise. To support these efforts, Salk Institute ...
Plants & Animals
Apr 24, 2024
2
32

While we go about our daily lives on Earth, a nuclear-powered robot the size of a small car is trundling around Mars looking for fossils. Unlike its predecessor Curiosity, NASA's Perseverance rover is explicitly intended ...
Astrobiology
Apr 24, 2024
4
12

Planetary nebula are some of nature's most stunning visual displays. The name is confusing since they're the remains of stars, not planets. But that doesn't detract from their status as objects of captivating beauty and intense ...
Astronomy
Apr 24, 2024
0
40
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, CO2− 3. The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C(=O)(O–)2.
The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverages — either by the addition of carbon dioxide gas under pressure, or by dissolving carbonate or bicarbonate salts into the water.
In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, CO2− 3. Carbonate minerals are extremely varied and ubiquitous in chemically-precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well as the main component of mollusc shells and coral skeletons); dolomite, a calcium-magnesium carbonate CaMg(CO3)2; and siderite, or iron (II) carbonate, FeCO3, an important iron ore. Sodium carbonate ("soda" or "natron") and potassium carbonate ("potash") have been used since antiquity for cleaning and preservation, as well as for the manufacture of glass. Carbonates are widely used in industry, e.g. in iron smelting, as a raw material for Portland cement and lime manufacture, in the composition of ceramic glazes, and more.
This text uses material from Wikipedia, licensed under CC BY-SA