This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Quantum state mixing in photobiology: New insight from ultrafast terahertz Stark spectroscopy
The membrane protein bacteriorhodopsin is a proton pump, in which proton transport is initiated by the light-induced isomerization of the chromophore retinal. The molecular quantum states involved in this ultrafast reaction have now been characterized by measuring their electric dipole moment.
The novel method of terahertz Stark spectroscopy reveals a mixing of electronically excited states with a direct impact on pathway and dynamics of the photoreaction.
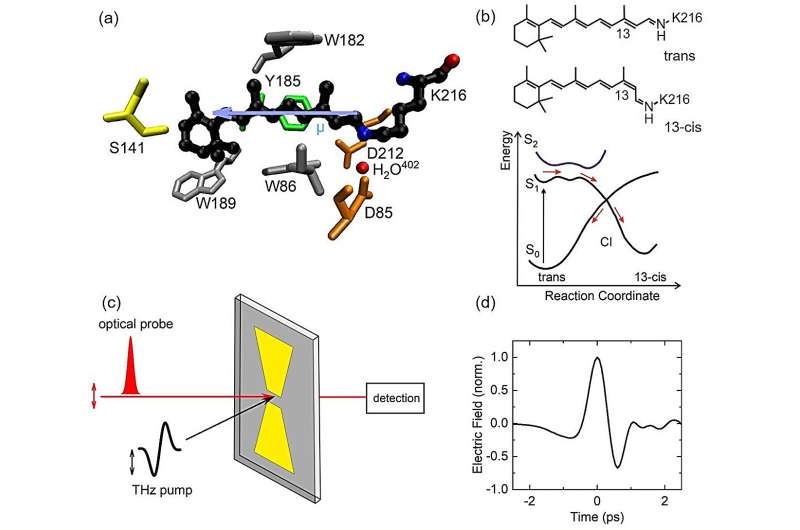
The protonated retinal Schiff base, the chromophore of bacteriorhodopsin, undergoes an ultrafast change of its molecular structure upon absorption of light. Photoexcitation promotes the chromophore to a particular range of its excited-state potential energy surface, from which the excited molecule evolves along a reaction coordinate to the intersection point of the excited and ground-state potential surfaces. After this early propagation in the excited state, isomerization occurs upon passing this crossing point within some 500 fs=5×10-13 s after excitation.
So far, the character of the excited-state potential governing reaction dynamics has remained controversial. Theoretical models have invoked either the first excited state S1 only or a mixed quantum state with a contribution from the second excited state S2.
This issue calls for new experimental insight into the excited-state character. A promising quantity to probe is the electric dipole moment of retinal, which is markedly different in the ground state S0 and the first and second excited states S1 and S2. Thus, a measurement of dipole change upon photoexcitation should allow for clarifying the character of the excited state relevant for the early dynamics of bacteriorhodopsin.
Applying the novel method of terahertz (THz) Stark spectroscopy, researchers from the Max-Born-Institut and Humboldt Universität in Berlin and the Ludwig Maximilians Universität in Munich have now determined the retinal electric dipole changes in bacteriorhodopsin (1 THz = 1012 Hz = 1012 oscillations per second).
As they report in the Proceedings of the National Academy of Sciences, photoexcitation results in a moderate change of the retinal dipole by some 5 Debye (1.67×10-29 CoulombMeter), much smaller than predicted for a neat S1 character of the excited state.
In contrast, their data and theoretical analysis show that admixture of the S2 state and temporal averaging over the first 120 fs of the ultrafast excited state dynamics account for the measured dipole change. Such results support a picture of pronounced quantum state mixing in the early electronic and nuclear dynamics of bacteriorhodopsin.
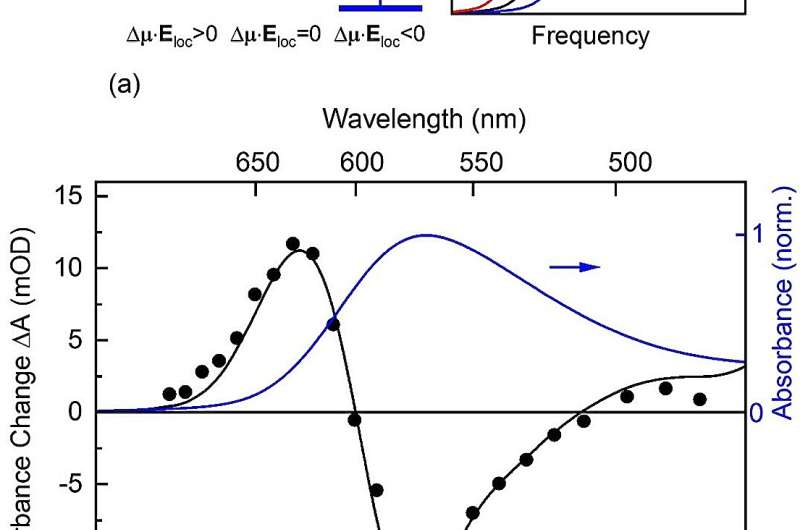
THz Stark spectroscopy employs a pump-probe approach, in which a THz pump pulse of a 1-ps duration (1 ps = 10-12 s) provides a strong external electric field, which induces a spectral (Stark) shift of the optical transitions from the retinal ground to the excited state.
This shift is proportional to the dipole difference ∆µ between the ground and excited state. The resulting absorption change of the sample is measured by a femtosecond probe pulse, which is short compared to the THz pulse and, thus, probes the momentary impact of the THz field. For a sample with a random spatial orientation of retinal chromophores, one observes a spectral broadening of the electronic absorption band, from which the dipole change ∆µ is derived.
In time, the broadening follows the intensity of the ultrashort THz pulse. On this ultrashort time scale, the protein environment of the chromophore is practically frozen, with a negligible impact of protein dynamics on the experimental observables.
In this way, THz Stark spectroscopy allows for the accurate measurement of dipole moments in molecular systems relevant to chemistry and biology.
More information: Jia Zhang et al, Ultrafast terahertz Stark spectroscopy reveals the excited-state dipole moments of retinal in bacteriorhodopsin, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2319676121
Journal information: Proceedings of the National Academy of Sciences
Provided by Max Born Institute for Nonlinear Optics and Short Pulse Spectroscopy (MBI)