This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New study unveils how water dynamics slow down at low temperatures
A scientist at the Institute for Molecular Science has published a study that provides insight into the puzzling phenomenon of dynamic slowdown in supercooled water, an essential step toward understanding the glass transition in liquids.
The study, "Unraveling the dynamic slowdown in supercooled water: The role of dynamic disorder in jump motions," explores the microscopic mechanisms that govern the dynamic behavior of water when it is cooled below its freezing point without forming ice. The study is published in The Journal of Chemical Physics.
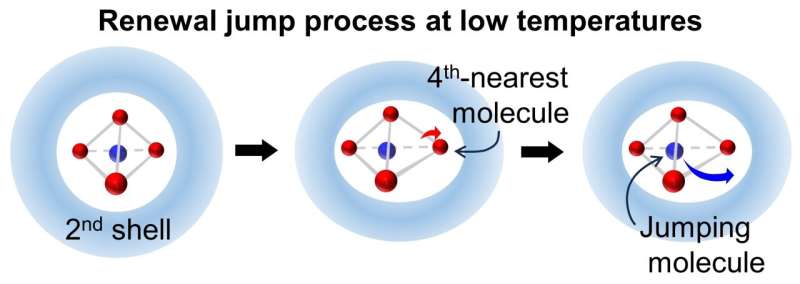
When water is supercooled, it exhibits a significant dynamic slowdown without any apparent structural changes. In this research, the jump dynamics of water molecules, which are elementary processes for structural changes, are studied using molecular dynamics simulations. The results show that these dynamics deviate from the expected Poisson statistics due to dynamic disorder as the temperature decreases.
Dynamic disorder refers to the competition between slow variables and the jump motions of molecules. The researcher identified the displacement of the fourth-nearest oxygen atom of a jumping molecule as the slow variable competing with the jump motion at lower temperatures. This displacement takes place in a fluctuating environment beyond the first hydration shell and profoundly affects the jump dynamics.
As the temperature decreases, the dynamics of water molecules become increasingly slow and intermittent, as the molecules are trapped within extended, stable, low-density domains. With further cooling, the interactions between molecules become more cooperative, increasing the complexity and dimensionality of the jump dynamics.
This research deepens our understanding of supercooled water and provides a foundation for future studies of the molecular dynamics of liquids approaching glass transitions. Glass transition processes are relevant in a wide range of applications.
Therefore, the application of the methods developed in this study will provide insight into how the slow motion of various materials can lead to glass transitions. Furthermore, this study paves the way for future research to elucidate the complex dynamics in other systems, such as proteins.
More information: Shinji Saito, Unraveling the dynamic slowdown in supercooled water: The role of dynamic disorder in jump motions, The Journal of Chemical Physics (2024). DOI: 10.1063/5.0209713
Provided by National Institutes of Natural Sciences