This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Regulatory hurdles for updating breakpoints for antimicrobial susceptibility test devices: What to know
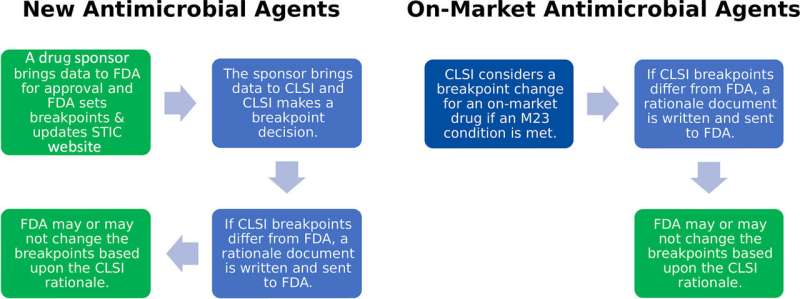
The unique regulatory environment for the clearance and use of antimicrobial susceptibility test (AST) systems is complex in the U.S. While members of the medical microbiology community recognize the importance of updating breakpoints for clinical and public health, there is a knowledge gap regarding the regulatory requirements for breakpoint updates and how these requirements impact the medical laboratory's update processes.
What do medical laboratory professionals need to know about the Food and Drug Administration's (FDA) breakpoint clearance process and how it impacts their testing?
Device and breakpoint clearance at the FDA
Two branches of the FDA are involved in clearing breakpoints for AST devices. The FDA Center for Drug Evaluation and Research (CDER) regulates antimicrobials and their associated breakpoints. In contrast, the FDA Center for Devices and Radiological Health regulates AST devices, including breakpoint implementation based on CDER-recommended breakpoints at the time of device clearance.
Medical devices are classified into 3 categories, and the regulatory requirements increase as the classification moves from class 1–3. These categories are primarily based on the device's intended use, indications for use and risk to the patient or user.
Antimicrobial susceptibility test devices are considered class 2 devices. For a new class 2 device to receive clearance or for a modification of the existing device to be cleared, a document called a 510(k) must be submitted to the FDA for review. According to the FDA, "a 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device."
In the case of breakpoints, the device manufacturer might have to submit data in the form of a 510(k) demonstrating acceptable drug performance on the device when using new breakpoints. To streamline and expedite the breakpoint update pathway, in 2019, the FDA created a process where manufacturers could submit a breakpoint change protocol with their 510(k) submission for clearance.
The purpose of this protocol was to allow manufacturers to proactively describe the actions they would take to update breakpoint information in the product label and, if conditions were appropriate, would prevent them from submitting another regulatory application to the FDA at the time of a breakpoint update. Although this was a helpful modification, limitations on how the protocol could be used still existed.
In Sept. 2023, the FDA issued a new, more comprehensive guidance document for AST manufacturers that supersedes the guidance document published by the agency in 2009 and provides an option for further expediting breakpoint updates, called a predetermined change control plan (PCCP), formerly the breakpoint control plan.
The PCCP may be pursued by manufacturers submitting an original 510(k) clearance document for their device (e.g., a new device coming to market) and those updating breakpoints on a previously cleared (legacy) device.
Once the PCCP is cleared with a 510(k) submission, in the event of future breakpoint updates, and if certain criteria are met, the label can be updated without an additional 510(k) submission to the FDA. The primary advantage of this program is that manufactures of new and legacy AST devices can re-process existing data to demonstrate acceptable performance with the new breakpoint, send that update to the FDA and update their label for use on the system. This revised process saves a significant amount of time because a review of data by the FDA is not required.
It is important to note that the breakpoint update should not significantly change the performance of the previously cleared device, and for a PCCP to be applicable, the device classification regulation, product code, intended use and technological classifications should be the same as those originally cleared.
Additionally, breakpoint updates are typically followed by customer letters, other forms of communication from the manufacturer that must be constructed and software updates. Given these considerations, medical laboratory professionals can expect a lag between the publication of new breakpoints and the full implementation of the breakpoints on their automated system, even if the clearance process is expedited.
Finally, manufacturers may only use the PCCP process for breakpoints published on the FDA STIC website, so this process only applies to CLSI breakpoints that the FDA has recognized.
New breakpoints, fewer organism indications
Before 2007, both FDA and Clinical and Laboratory Standards Institute (CLSI) breakpoints could be reported from an FDA-cleared AST device. In 2007, the FDA published a class II special guidance document specifically for AST systems, which required that AST devices in the U.S. only report FDA-recognized breakpoints, and the use of CLSI breakpoints was allowed only for instruments with clearances in 2007 or earlier.
After a revision in 2009, the document stated that results from AST devices should be reported only for microbial species that had in vivo efficacy data listed in the antimicrobial's instructions-for-use. Today, this poses a dilemma for AST device manufacturers seeking clearance for new breakpoints on their systems, and for medical laboratories hoping to update them. "Grandfathering" of old breakpoints or broad organism claims is no longer allowed.
This means device manufacturers may only receive clearance for breakpoints recognized by the FDA and for organisms claimed in the original antimicrobial label. Additionally, the list of organisms with in-vivo data in the drug label (known as "list 1") is typically limited and often does not contain all clinically relevant organisms that may be treated in the clinical setting.
These regulations significantly impact medical laboratories for several reasons. For example, many clearances were obtained before 2007 for large organism categories like "clinically significant gram-negative bacilli." Because of this, performing susceptibility testing (using the cleared device) on all clinically significant gram-negative bacilli was considered on-label.
Under the new FDA regulations, many organism claims may be removed when clearance for new breakpoints is pursued, making the reporting of any organism not listed in the test package insert off-label.
Let's walk through an example using the antibiotic cefazolin. The breakpoints for cefazolin were lowered by CLSI in 2010, and FDA-CDER agrees with these breakpoints for systemic infections (not uncomplicated urinary tract infections).
Since the FDA recognizes the CLSI breakpoints, AST device manufacturers can submit data demonstrating the performance of, and seek clearance for, those breakpoints on their device. However, since the organism claims must now reflect what is in the cefazolin antibiotic package insert, the broad category of "clinically significant gram-negative bacilli" no longer applies.
In the case of cefazolin, the only gram-negative organisms listed in the label for systemic infection(s) are Escherichia coli and Proteus mirabilis. Manufacturers are allowed to submit data that supports the testing of non "list 1" organisms, as long these organisms do not comprise more than 5% of the dataset and are biologically similar to the "list 1" organisms (e.g., are all Enterobacterales).
Whether or not a package insert will contain only the species listed in the drug label or a larger group like Enterobacterales will depend on the data submitted by the manufacturer. Regardless, this regulation may limit the species indicated in the label since including large groups with off-label organisms (e.g., clinically significant gram-negative bacilli) is no longer allowed.
If a medical laboratory wants to use the new breakpoints on their device for other gram-negative organisms, this is considered off-label, and the laboratory has to perform a validation. If the manufacturer has performed an internal validation for additional organism-drug combinations, these data may be applicable in countries that follow ISO 20776-2 guidance for determining the performance of AST systems.
This is because ISO performance standards assess essential agreement and bias and are independent of breakpoints, which allows new breakpoints to be implemented immediately. However, this differs in the U.S., where device manufacturers must strictly adhere to FDA regulations.
What this means for medical laboratories
Updating breakpoints is essential for appropriate patient care and public health and should be prioritized by medical microbiology laboratories. While this process may be a significant undertaking, multiple resources are available to assist laboratories and help them maintain updated AST breakpoints in all their testing methodologies.
In a recent publication summarizing the ASM 2022 Clin Micro Open conference, published in the Journal of Clinical Microbiology, the authors note a significant lack of understanding regarding barriers and needs between industry, regulatory, clinical and public health microbiology groups. The publication calls for greater group dialogue and collaboration between sectors to address breakpoint updating and implementation barriers.
Medical laboratory professionals may find themselves performing numerous off-label validations to use updated breakpoints on their device or to validate organisms now considered off-label after a recent breakpoint clearance.
Users of AST devices must contact the manufacturer with any questions about clearance or breakpoint updates to understand what may require validation and plan accordingly. Additionally, it is imperative to collaborate with clinical colleagues to prioritize breakpoint and organism validations based on need, resources and local epidemiology.
More information: Jean B. Patel et al, Updating breakpoints in the United States: a summary from the ASM Clinical Microbiology Open 2022, Journal of Clinical Microbiology (2023). DOI: 10.1128/jcm.01154-22
Journal information: Journal of Clinical Microbiology
Provided by American Society for Microbiology